二甲氨亚甲基丙二酸二乙酯 | 18856-68-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:176-178 °C(Solv: ethanol (64-17-5))
-
沸点:134-136°C 0,5mm
-
密度:1.069±0.06 g/cm3(Predicted)
-
闪点:134-136°C/0.5mm
-
溶解度:溶于甲醇。
-
稳定性/保质期:
常温常压下稳定,避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:15
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:55.8
-
氢给体数:0
-
氢受体数:5
安全信息
-
海关编码:2922499990
-
储存条件:常温下应密闭避光保存,并保持通风和干燥。
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (羰基甲基氨)亚甲基丙二酸二乙酯 diethyl 2-(1-carboxymethylaminomethylene)propane-1,3-dicarboxylate 54132-81-9 C10H15NO6 245.232 —— 2-(benzylamino-methylene)-malonic acid diethyl ester 54535-21-6 C15H19NO4 277.32
反应信息
-
作为反应物:描述:参考文献:名称:Quinolin-4(1H)-imines are Potent Antiplasmodial Drugs Targeting the Liver Stage of Malaria摘要:We present a novel series of quinolin-4(1H)-imines as dual-stage antiplasmodials, several-fold more active than primaquine in vitro against Plasmodium berghei liver stage. Among those, compounds 5g and 5k presented low nanomolar IC50 values. The compounds are metabolically stable and modulate several drug targets. These results emphasize the value of quinolin-4(1H)-imines as a new chemotype and their suitable properties for further drug development.DOI:10.1021/jm400246e
-
作为产物:描述:参考文献:名称:制备喹啉类化合物的方法摘要:本发明涉及喹啉类化合物的制备领域,公开了一种制备式(I)所示的喹啉类化合物的方法,该方法包括:在惰性高沸点多醚溶剂的存在下,使式(III)所示的化合物与3,5‑二氯苯胺进行取代反应得到式(II)所示的化合物,之后经关环反应得到式(I)所示的化合物,其中,X1、X3、X4、X5和X6各自独立地为‑CN或‑COOR’,R’为C1‑C4的烷基;其中,X1与X3、X4、X5和X6中的至少一个相同;X3和X4相同或不同,X5和X6相同或不同;X2为羟基或氨基;R1和R2各自独立地为氢或C1‑C4的烷基;R1和R2相同或不同。本发明提供的方法具有反应温度较低、成本低且收率较高的优势,利于工业化。公开号:CN114621141A
文献信息
-
一种噁喹酸的合成方法
-
Vicinal Tricarbonyl products from singlet oxygen reactions.作者:Harry H. Wasserman、William T. HanDOI:10.1016/0040-4039(84)80120-3日期:1984.1Vicinal tricarbonyl systems are readily formed by reacting β-dicarbonyl precursors with DMF acetal to form enamines which are then cleaved by photooxidation. This procedure may be applied to the formation of carbacephams.
-
ENAMINONES AS BUILDING BLOCKS IN ORGANIC SYNTHESIS: SYNTHESIS OF NEW POLYFUNCTIONAL PYRIDINES, CONDENSED PYRIDINES, AND PENTA SUBSTITUTED BENZENE作者:F. A. Abu-Shanab、Y. M. Elkholy、M. H. ElnagdiDOI:10.1081/scc-120014783日期:2002.1ABSTRACT Several new thienopyridine and methylthioether derivatives have been synthesized. New synthesis of pyrido[2,3-b]-thieno[3,2-d]pyrimidine and penta substituted benzene were achieved.
-
The synthesis of some dialkyl 4-(3-substituted amino)phenyl-1,4-dihydro-2,6-dimethylpyridine-3,5-dicarboxylates作者:Igor Simonič、Branko StanovnikDOI:10.1002/jhet.5570340614日期:1997.11Dialkyl 4-(3-aminophenyl)-1,4-dihydro-2,6-dimethylpyridine-3,5-dicarboxylates 1 were transformed into alkyl 4-(3-(((2-benzoylamino-2-methoxycarbonyl)ethenyl)amino)phenyl)-1,4-dihydro-2,6-dimethyl-pyridine-3,5-dicarboxylates 4 and with 2,2-disubstituted-1-dimethylaminoethenes 7 into dimethyl 4-(3-(((2,2-diacyl- or 2-acyl-2-alkoxycarbonyl)ethenyl)amino)phenyl)-1,4-dihydro-2,6-dimethylpyridine-3,5-dicarboxylates
-
Synthesis and Cytotoxicity of Thieno[2,3-b]Pyridine Derivatives Toward Sensitive and Multidrug-Resistant Leukemia Cells作者:Salah A. Al-Trawneh、Amer H. Tarawneh、Anastassiya V. Gadetskaya、Ean-Jeong Seo、Mohammad R. Al-Ta’ani、Samir A. Al-Taweel、Mustafa M. El-AbadelahDOI:10.17344/acsi.2020.6609日期:——
A new series of substituted ethyl 7-cyclopropyl-2-(2-aryloxo)-3-nitro-4-oxo-4,7-dihydrothieno[2,3-b]pyridine-5-carboxylates 3a–e were prepared by utilizing ethyl 2-chloro-7-cyclopropyl-3-nitro-4-oxo-4,7-dihydrothieno[2,3-b]pyridine-5-carboxylate (1) and replacing of the 2-chlorine with anions obtained from phenol (2a), salicylaldehyde derivatives 2b–d or thiophenol (2e), leading to the respective ethyl 7-cyclopropyl-2-(2-aryloxo)-3-nitro-4-oxo-4,7-dihydrothieno[2,3-b]pyridine-5-carboxylates 3a–e. The new compounds were evaluated for their in vitro cytotoxicity towards sensitive CCRF-CEM and multidrug-resistant CEM/ADR5000 leukemia cells. The screening revealed that compounds 3a, 3b, and 3e inhibited the growth of both cell lines. Compound 3b, with a phenol moiety, exhibited the highest growth inhibitory activity against CEM/ADR5000 and CCRF-CEM cells with IC50 values 4.486 ± 0.286 and 2.580 ± 0.550 μM, respectively. Collectively, the presented results demonstrate that the synthesized thieno[2,3-b]pyridines warrant further exploration for potential use as anti-cancer agents.
一系列新的取代基乙基7-环丙基-2-(2-芳氧基)-3-硝基-4-氧代-4,7-二氢噻吩[2,3-b]吡啶-5-羧酸乙酯3a-e通过利用乙基2-氯-7-环丙基-3-硝基-4-氧代-4,7-二氢噻吩[2,3-b]吡啶-5-羧酸乙酯(1)并用苯酚(2a)、水杨醛衍生物2b-d或硫酚(2e)获得的阴离子替换2-氯而制备。这导致相应的乙基7-环丙基-2-(2-芳氧基)-3-硝基-4-氧代-4,7-二氢噻吩[2,3-b]吡啶-5-羧酸乙酯3a-e。对这些新化合物在体外对敏感的CCRF-CEM和多药耐药CEM/ADR5000白血病细胞的细胞毒性进行了评估。筛选结果显示,化合物3a、3b和3e抑制了两种细胞系的生长。具有酚基的化合物3b对CEM/ADR5000和CCRF-CEM细胞表现出最高的生长抑制活性,IC50值分别为4.486 ± 0.286和2.580 ± 0.550 μM。总的来说,所呈现的结果表明,合成的噻吩[2,3-b]吡啶值得进一步探索,作为抗癌药物的潜在用途。
表征谱图
-
氢谱1HNMR
-
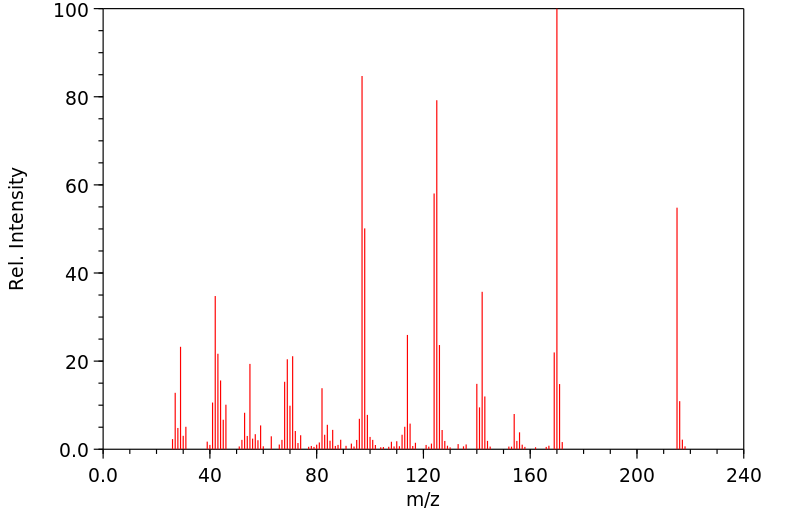
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息