邻苯二甲酸二辛酯 | 82208-43-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-50 °C
-
沸点:386 °C (lit.)
-
密度:0.985 g/mL at 20 °C (lit.)
-
蒸气密度:>16 (vs air)
-
闪点:405 °F
-
溶解度:与矿物油和己烷混溶 (US EPA, 1985)
-
介电常数:5.1(24℃)
-
暴露限值:Potential occupational carcinogen. NIOSH REL: TWA 5, STEL 10, IDLH 5,000; OSHA PEL: TWA 5; ACGIH TLV: TWA 5 (adopted).
-
LogP:7.5 at 20℃
-
物理描述:Di(2-ethylhexyl) phthalate is a colorless to pale yellow oily liquid. Nearly odorless. (USCG, 1999)
-
颜色/状态:Liquid
-
气味:Slight odor
-
蒸汽密度:13.45 (NTP, 1992) (Relative to Air)
-
蒸汽压力:1.42X10-7 mm Hg at 25 °C
-
稳定性/保质期:
-
自燃温度:735 °F (390 °C)
-
分解:When heated to decomp it emits acrid smoke.
-
粘度:22 cSt at 20 °C; 386 cSt at 0 °C; 5 cSt at 100 °C
-
燃烧热:-15,130 BTU/LB= -8410 CAL/G= -352X10+5 JOULES/KG
-
表面张力:LIQUID SURFACE TENSION: EST 15 DYNES/CM= 0.015 N/M @ 20 °C LIQUID-WATER INTERFACIAL TENSION: EST 30 DYNES/CM= 0.03 N/M @ 20 °C.
-
折光率:Index of refraction: 1.4853 at 20 °C/D
-
碰撞截面:212.2 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)]
-
保留指数:2499.4;2499;2499;2505;2492.6;2507;2509;2488;2498;2516.7;2504;2505;2506;2480;2505;2506;2509;2506;2507;418.6;418.7;406.14;406.15
计算性质
-
辛醇/水分配系数(LogP):7.4
-
重原子数:28
-
可旋转键数:16
-
环数:1.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
ADMET
安全信息
-
职业暴露等级:B
-
职业暴露限值:TWA: 5 mg/m3, STEL: 10 mg/m3
-
TSCA:Yes
-
危险等级:9
-
立即威胁生命和健康浓度:5,000 mg/m3
制备方法与用途
无色透明液体,具有特殊气味。不溶于水,能溶解在大多数有机溶剂和烃类中,并与多数工业用树脂有良好的相容性。它还与醋酸纤维素、聚醋酸乙烯酯部分相容。
用途该物质广泛用作塑料的主增塑剂,特别是用于聚氯乙烯制品中。此外,它可以作为DOP(邻苯二甲酸二辛酯)的替代品,在增塑糊中表现出良好的粘度稳定性。它还可用作气相色谱固定剂和增塑剂。
该品是最广泛使用的增塑剂之一,除乙酸纤维素、聚乙酸乙烯外,与绝大多数工业上使用的合成树脂和橡胶均具有良好相容性。产品具有综合性能良好、混合性能优良、增塑效率高且挥发性较低等特点,低温柔软性较好,耐水抽出,电气性能及耐热性和耐候性也十分优异。
这种主增塑剂广泛应用于聚氯乙烯各种软质制品的加工,例如薄膜、薄板、人造革、电缆料和模塑品等。由于无毒特性,该产品可用于与食物接触的包装材料;但因其易被脂肪抽出,不宜用于脂肪性食品包装材料。此外,它还可用作硝基纤维素漆,使漆膜具有较高的抗张强度。在多种合成橡胶中,此物质亦有良好的软化作用。
气相色谱固定液方面,该品可应用于最高使用温度为150℃(以甲醇为主、二氯甲烷为溶剂)的条件,用于选择性保留和分离芳香族化合物、不饱和化合物及各种含氧化物(如醇、醛、酮及酯等)。它还用作增塑剂。
生产方法目前,增塑剂的生产技术主要分为两大类:主增塑剂的大规模连续化生产和特殊增塑剂的小批量间歇生产。其中,邻苯二甲酸酯类增塑剂占据消费量的80%以上,因此出现了以邻苯二甲酸二辛酯为中心的连续化大生产。
在工业生产中,邻苯二甲酸酯是通过苯酐与相应的醇(如2-乙基己醇或异辛醇)在硫酸、对甲苯磺酸等酸性催化剂存在下进行酯化反应制得。非酸性催化剂如钛酸酯、氧化亚锡、铝酸盐等也可以用于该过程。
具体原料消耗定额为:苯酐383千克/吨、2-乙基己醇(95%)671千克/吨、硫酸6千克/吨和纯碱10千克/吨。
类别与性质 农药类别本品归类于农药范畴。
毒性分级毒性级别为中毒。急性毒性方面,大鼠口服LD50值为30000毫克/公斤,小鼠口服LD50值为1500毫克/公斤。
刺激数据皮肤接触(兔子):500毫克/24小时,轻度刺激;眼睛接触(兔子):500毫克,轻度刺激。
爆炸物危险特性与空气混合后可发生爆炸;与氧化剂反应强烈。
可燃性危险特性易燃,在火场会产生辛辣刺鼻的烟雾。
储运特性应储存在低温、通风且干燥的地方。
灭火方法 职业卫生标准时间加权平均浓度(TWA)不超过5毫克/立方米,短期暴露极限值(STEL)不超过10毫克/立方米。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 邻苯二甲酸单乙基己基酯 (2-ethylhexyl) hydrogen phthalate 4376-20-9 C16H22O4 278.348 邻苯二甲酸单丁酯 phthalic acid monobutyl ester 131-70-4 C12H14O4 222.241 邻苯二甲酸丁苄酯 Butyl benzyl phthalate 85-68-7 C19H20O4 312.365 邻苯二甲酸 benzene-1,2-dicarboxylic acid 88-99-3 C8H6O4 166.133 苯酐 phthalic anhydride 85-44-9 C8H4O3 148.118 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 邻苯二甲酸单乙基己基酯 (2-ethylhexyl) hydrogen phthalate 4376-20-9 C16H22O4 278.348 邻苯二甲酸 benzene-1,2-dicarboxylic acid 88-99-3 C8H6O4 166.133
反应信息
-
作为反应物:参考文献:名称:有机酸辅助制备羟基磷灰石负载镍芳烃加氢催化剂摘要:探索有机酸结构变化对催化剂性能的影响揭示了增强芳烃加氢活性的重要见解。以羟基磷灰石为载体,并辅以最佳配比的羟基二元羧酸(酒石酸)的Ni催化剂,可产生相对较小的Ni纳米粒子、较高的Ni 0比例和增加的酸性位点,表现出优异的加氢性能。DOI:10.1002/cctc.202400208
-
作为产物:描述:alkaline earth salt of/the/ methylsulfuric acid 生成 邻苯二甲酸二辛酯参考文献:名称:Complete Study of the Pyrolysis and Gasification of Scrap Tires in a Pilot Plant Reactor摘要:The pyrolysis and gasification of tires was studied in a pilot plant reactor provided with a system for condensation of semivolatile matter. The study comprises experiments at 450, 750, and 1000 degreesC both in nitrogen and 10% oxygen atmospheres. Analysis of all the products obtained (gases, liquids, char, and soot) are presented. In the gas phase only methane and benzene yields increase with temperature until 1000 degreesC. In the liquids the main components are styrene, limonene, and isoprene. The solid fraction (including soot) increases with temperature. Zinc content of the char decreases with increasing temperature.DOI:10.1021/es034608u
-
作为试剂:参考文献:名称:Asymmetric epoxidation of styrene derivatives by styrene monooxygenase from Pseudomonas sp. LQ26: effects of α- and β-substituents摘要:Recombinant Escherichia coli expressing a styrene monooxygenase, StyAB2, from Pseudomonas sp. LQ26 was applied to synthesize a range of chiral epoxides from conjugated styrene derivatives with excellent (>99%) enantioselectivity in most cases. The substrate preference was studied with a special focus on the steric effect of alpha- and beta-substituents. (C) 2011 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetasy.2010.12.022
文献信息
-
[EN] PHENOTHIAZINE DERIVATIVES AND USES THEREOF<br/>[FR] DÉRIVÉS DE PHÉNOTHIAZINE ET LEURS UTILISATIONS申请人:CAMP4 THERAPEUTICS CORP公开号:WO2019195789A1公开(公告)日:2019-10-10The present invention provides phenothiazine compounds, processes for their preparation, pharmaceutical compositions comprising the compounds, and the use of the compounds or the compositions in the treatment of various diseases or conditions, for example ribosomal disorders and ribosomopathies, e.g. Diamond Blackfan anemia (DBA).
-
[EN] PROCESS FOR PREPARING CARBOXYLIC ACID ESTERS IN THE PRESENCE OF A TITANIUM-CONTAINING CATALYST<br/>[FR] PROCÉDÉ DE PRÉPARATION D'ESTERS D'ACIDES CARBOXYLIQUES EN PRÉSENCE D'UN CATALYSEUR CONTENANT DU TITANE申请人:SIBUR HOLDING PUBLIC JOINT STOCK CO公开号:WO2016043616A1公开(公告)日:2016-03-24The present invention relates to a process for preparing carboxylic acid esters, comprising esterification of a carboxylic acid with an alcohol in the presence of a titanium-containing catalyst selected from compounds of a general formula, Tin(OR)x(OR')xOy, wherein n is an integer from 1 to 4; у is an integer from 0 to 6; x can be the same or different and is an integer from 2 to 8; R is a linear or branched C1-C18alkyl, C3-C18cycloalkyl, R' is aryl optionally comprising an electron-donor substituent; or a mixture thereof, with the proviso that if n is 1, then x is 2 and у is 0; and if n>1, then the compounds comprise at least two alkoxy groups and two aryloxy groups. The present invention also relates to a process for preparing carboxylic acid esters, wherein a compound of general formula (I) or (II), wherein q represents an integer of 1 to 4; Y is independently R or R'; or a mixture thereof, with the proviso that the compounds comprise at least two alkoxy groups and two aryloxy groups, is used as a catalyst. The claimed process allows to reduce the amount of a used catalyst and the time of the process duration, while increasing the conversion rate of initial reagents and the yield of a target product.本发明涉及一种制备羧酸酯的方法,包括在存在选择自通式Tin(OR)x(OR')xOy的化合物的钛催化剂的情况下,将羧酸与醇酯化,其中n为1至4的整数;у为0至6的整数;x可以相同也可以不同,为2至8的整数;R为线性或支链的C1-C18烷基,C3-C18环烷基,R'为芳基,可包含电子给体取代基;或二者的混合物,但若n为1,则x为2且у为0;若n>1,则化合物至少包含两个烷氧基和两个芳氧基。本发明还涉及一种制备羧酸酯的方法,其中通式(I)或(II)的化合物,其中q表示1至4的整数;Y独立地为R或R';或二者的混合物,但化合物至少包含两个烷氧基和两个芳氧基,被用作催化剂。所述方法允许减少使用的催化剂量和过程持续时间,同时提高初始试剂的转化率和目标产品的产率。
-
Process for the preparation of olmesartan medoxomil申请人:KRKA, tovarna zdravil, d.d., Novo mesto公开号:EP1816131A1公开(公告)日:2007-08-08The present invenion relates to an improved process for the manufacture of olmesartan and pharmaceutically acceptable salts and esters thereof as an active ingredient of a medicament for the treatment of hypertension and related diseases and conditions.本发明涉及一种改进的工艺,用于制造奥美沙坦及其药用可接受的盐和酯,作为治疗高血压及相关疾病和症状的药物的活性成分。
-
[EN] HEMI-AMINAL ETHERS AND THIOETHERS OF N-ALKENYL CYCLIC COMPOUNDS<br/>[FR] ÉTHERS ET THIOÉTHERS HÉMIAMINAUX DE COMPOSÉS CYCLIQUES N-ALCÉNYLIQUES申请人:ISP INVESTMENTS INC公开号:WO2014116560A1公开(公告)日:2014-07-31Described herein are hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds that may be produced through a reaction comprising: (A) at least one first reactant represented by a structure (I), wherein X is a functionalized or unfunctionalized C1-C5 alkylene group optionally having one or more heteroatoms, and each R1, R2, and R3 is independently selected from the group consisting of hydrogen and functionalized and unfunctionalized alkyl groups optionally having one or more heteroatoms, and (B) at least one second reactant having at least one hydroxyl moiety or thiol moiety. The hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds may comprise a polymerizable moiety, in which case they may be left as-is or used to create homopolymers or non-homopolymers, or they may not comprise a polymerizable moiety. A wide variety of formulations may be created using the hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds, including personal care, oilfield, and construction formulations.
-
Hexahydrophthalate based compound and process for producing the same申请人:Shieh Sung-Yueh公开号:US20090281349A1公开(公告)日:2009-11-12A hexahydrophthalate based compound is adapted to use as a plasticizer that contains no phthalic acid and benzoic acid, possess physical properties superior to DEHA and DINA in transparency and adhesion and is friendly to organisms and the environment; and a process for producing the hexahydrophthalate based compound includes esterifying hexahydrophthalic anhydride, a diol, and a catalyst for decarboxylation to get hexahydrophthalic alcohol, and adding a monoacid into the hexahydrophthalic alcohol for further esterification, thereby obtaining the hexahydrophthalate based compound.
表征谱图
-
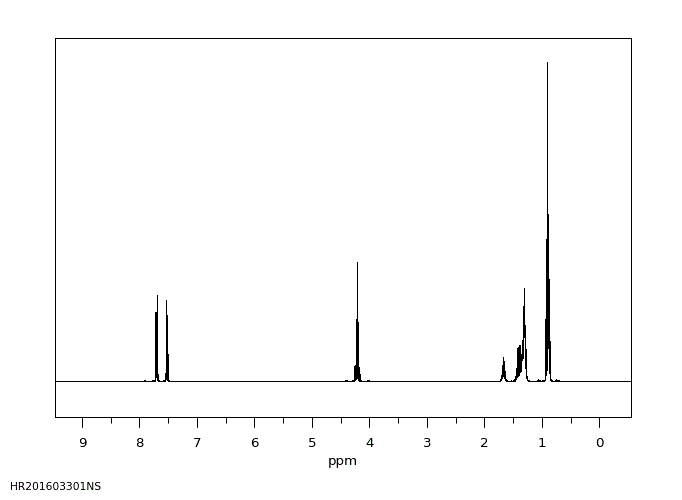
氢谱1HNMR
-
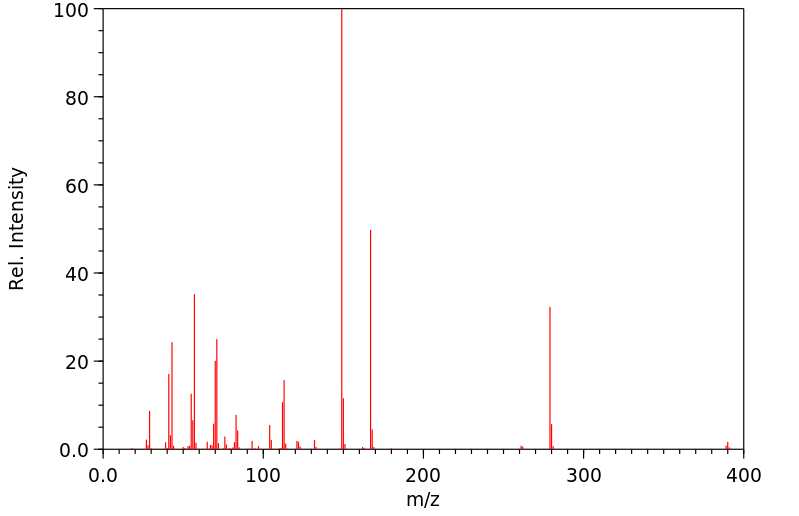
质谱MS
-
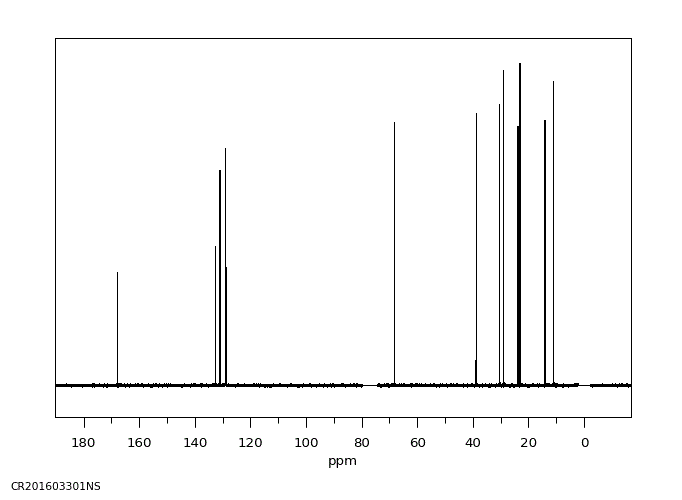
碳谱13CNMR
-
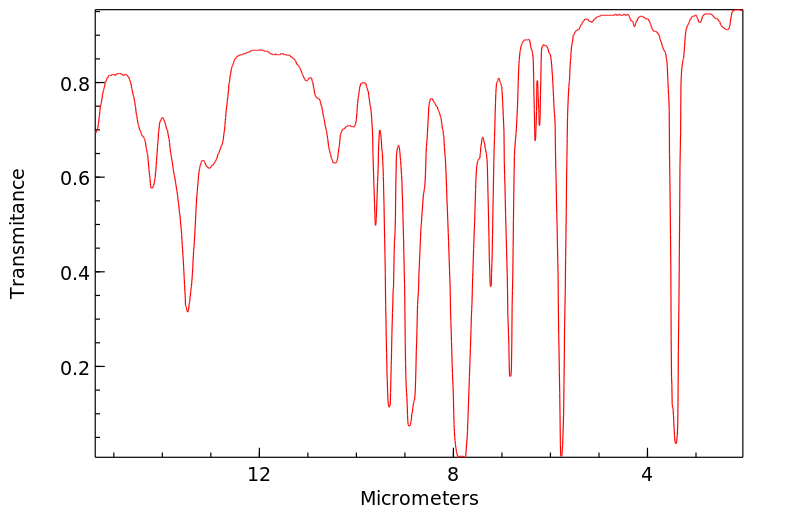
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息