酒石酸去氧肾上腺素 | 17162-39-9
中文名称
酒石酸去氧肾上腺素
中文别名
L-重酒石酸去氧肾上腺素;重酒石酸去氧肾上腺素;重酒石酸-L-苯肾上腺素;3-[(1R)-1-羟基-2-(甲基氨基)乙基]苯酚2,3-二羟基琥珀酸盐(1:1)
英文名称
Phenylephrine bitartrate
英文别名
2,3-dihydroxybutanedioic acid;3-[1-hydroxy-2-(methylamino)ethyl]phenol
CAS
17162-39-9;14787-58-7
化学式
C13H19NO8
mdl
——
分子量
317.29
InChiKey
NHKOTKKHHYKARN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
反应信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):-1.48
-
重原子数:22
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.38
-
拓扑面积:168
-
氢给体数:7
-
氢受体数:9
安全信息
-
海关编码:2922509090
-
WGK Germany:3
文献信息
-
Nasal and Ophthalmic Delivery of Aqueous Corticosteroid Solutions申请人:Pipkin James D.公开号:US20090312724A1公开(公告)日:2009-12-17The present invention is directed to methods of treating nasal and/or ophthalmic diseases, symptoms, or disorders that are therapeutically responsive to corticosteroid therapy by delivering aqueous solution formulations comprising a corticosteroid to nasal and ophthalmic tissues. The invention is also directed to methods, systems, devices, and compositions for delivering aqueous solution formulations comprising a corticosteroid and an antihistamine to nasal and ophthalmic tissues.
-
BENZONATATE MODIFIED RELEASE SOLID TABLETS AND CAPSULES申请人:TRIS Pharma Inc.公开号:US20160199341A1公开(公告)日:2016-07-14A 12-hour anti-tussive modified release solid tablet or capsule is described which comprises a benzonatate adsorbate in a matrix with a sufficient amount of one or more pharmaceutically acceptable modified release pH-independent, substances to provide a 12-hour modified release profile to the benzonatate, wherein there is substantially no benzonatate release from the tablet or capsule in the buccal cavity and no more than about 25% release of the benzonatate within 1 hour as determined in an in vitro dissolution assay.
-
Stable acetylsalicylic acid and diphenhydramine citrate effervescent composition申请人:MILES LABORATORIES, INC.公开号:EP0194547A2公开(公告)日:1986-09-17A substantially stable composition containing an effervescent couple, acetylsalicylic acid and diphenhydramine citrate is disclosed. Other known active agents, as well as optional ingredients such as flavorings, may also be included in the composition. The composition effervesces when placed m water The effervescent couple preferably is sodium bicarbonate and citric acid.
-
Skin penetration system for salts of amine-functional drugs申请人:THE PROCTER & GAMBLE COMPANY公开号:EP0351897A2公开(公告)日:1990-01-24The invention involves pharmaceutical compositions for topical application comprising: (a) a pharmaceutically-acceptable salt of addition of an amine-functional drug (other than opioid analgesic drugs); (b) a C₇ to C₂₂ straight-chain or branched-chain, saturated or unsaturated, fatty acid having a melting point of less than about 50°C; and (c) a C₃-C₄ alkane diol.
-
Effervescent cold or sinus allergy medicine composition having reduced sodium content申请人:BAYER AG公开号:EP0418564A1公开(公告)日:1991-03-27An effervescent cold or sinus allergy medicine composition having reduced sodium content is produced from a mixture of an analgesic, such as acetylsalicylic acid, acetaminophen, ketoprofen, or a mixture thereof, citric acid, sodium bicarbonate, calcium carbonate, potassium bicarbonate, antihistamine, decongestant, and minor amounts of flavors and sweeteners.
表征谱图
-
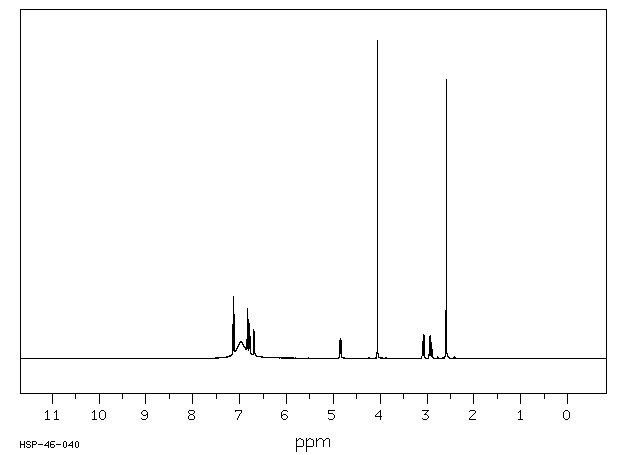
氢谱1HNMR
-
质谱MS
-
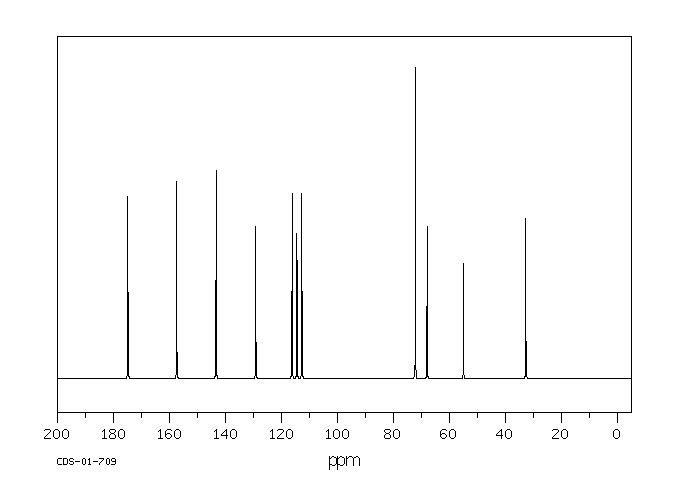
碳谱13CNMR
-
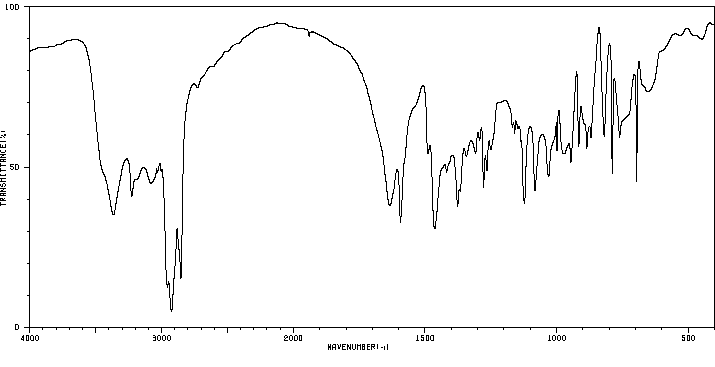
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷