反式-2-甲基-4-己烯-3-醇 | 96346-76-8
中文名称
反式-2-甲基-4-己烯-3-醇
中文别名
——
英文名称
(E)-2-methyl-4-hexen-3-ol
英文别名
trans-2-methyl-4-hexen-3-ol;(E)-2-methylhex-4-en-3-ol
CAS
96346-76-8
化学式
C7H14O
mdl
——
分子量
114.188
InChiKey
WFRYPJOHULJNDS-SNAWJCMRSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:141.9±8.0 °C(Predicted)
-
密度:0.839±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-己-4-烯-3-醇 (S)-2-Methyl-4(E)-hexen-3-ol 4798-60-1 C7H14O 114.188 —— (-)-(2E,4R)-5-methyl-2-hexen-4-ol 92283-72-2 C7H14O 114.188
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis of vinyl 1,2-diketones摘要:A new route is outlined for preparation of vinyl 1,2-diketones via a three-step sequence. First, allylic alcohols are photooxidized by O-2 to hydroperoxides, which are reduced to vinyl 1,2-diols. These vinyl 1,2-diols are oxidized to vinyl 1,2-diketones with oxoammonium salts, which are prepared in situ from organic nitroxyl radicals. The new route is short, avoids the use of protecting groups, and is generally applicable to obtain aliphatic or aromatic vinyl 1,2-diketones. (C) 2004 Published by Elsevier Ltd.DOI:10.1016/j.tetlet.2004.03.157
-
作为产物:描述:1-isopropylbutyn-2-ol 在 lithium aluminium tetrahydride 作用下, 生成 反式-2-甲基-4-己烯-3-醇参考文献:名称:在取代的烯丙基N,N-二烷基酰胺基亚砜的[2,3]-σ重排中的非对映选择性。[(1')S *,(S)S * ]-(2'E)-4-[[3'-(4''-溴苯基)-1'-甲基-2'-丙烯基的X射线分子结构[亚磺酰基]-吗啉摘要:通过与三个N,N-二烷基酰胺基亚磺酰氯2(在氮原子上具有代表性的R基团)反应,一些取代的仲E或Z烯丙基醇(1a-h)已转化为相应的非对映异构烯丙基亚磺酰胺(3 + -3'av),其比例已通过1 H NMR光谱测定。已经观察到五例完全非对映选择性[2,3]-σ重排的情况。DOI:10.1016/s0040-4020(01)82318-8
文献信息
-
Modulating the stereochemical outcome of the Ireland–Claisen reaction of (E)- and (Z)-allylic glycolates作者:Ken S. Feldman、Brandon R. SelfridgeDOI:10.1016/j.tetlet.2011.12.011日期:2012.2The diastereoselectivity of Ireland–Claisen rearrangements of allylic glycolates is dependent on the E:Z ratio of the silyl ketene acetals, the alkene geometry in the allyl unit, and the transition state topography. High yields and excellent diastereoselectivities (>95:5) have been achieved for selected substrates, including those with R2 = ethyl that results in a newly formed quaternary center. A
-
Palladium-Catalyzed γ-Selective and Stereospecific Allyl−Aryl Coupling between Acyclic Allylic Esters and Arylboronic Acids作者:Hirohisa Ohmiya、Yusuke Makida、Dong Li、Masahito Tanabe、Masaya SawamuraDOI:10.1021/ja9092264日期:2010.1.20Reactions between acyclic (E)-allylic acetates and arylboronic acids in the presence of a palladium catalyst prepared from Pd(OAc)(2), phenanthroline (or bipyridine), and AgSbF(6) (1:1.2:1) proceeded with excellent gamma-selectivity to afford allyl-aryl coupling products with E-configuration. The reactions of alpha-chiral allylic acetates took place with excellent alpha-to-gamma chirality transfer在由 Pd(OAc)(2)、菲咯啉(或联吡啶)和 AgSbF(6) (1:1.2:1) 制备的钯催化剂存在下,无环 (E)-烯丙基乙酸酯和芳基硼酸之间的反应进行得非常好γ-选择性提供具有 E-构型的烯丙基-芳基偶联产物。α-手性烯丙基乙酸酯的反应发生了具有顺式立体化学的优异的 α 到 γ 手性转移,以产生在苄基位置具有立体中心的烯丙基化芳烃。该反应在烯丙基乙酸酯和芳基硼酸中都可以耐受范围广泛的官能团。此外,肉桂醇衍生物的γ-芳基化得到含有未共轭烯基取代基的墒二芳基烷烃衍生物。该方法的合成效用通过其在 (+)-舍曲林(一种抗抑郁药)的有效合成中的应用得到证明。观察到的 γ-区域选择性和 E-1,3-syn 立体化学基于 Pd(II) 机制进行合理化,该机制涉及阳离子单(酰氧基)钯(II)配合物和芳基硼酸之间的金属转移,以及定向碳钯化,然后是 Syn-β -酰氧基消除。与可能的中间体相
-
Formation of Chiral Quaternary Carbon Stereocenters Using Silylene Transfer Reactions: Enantioselective Synthesis of (+)-5-<i>epi</i>-Acetomycin作者:Stacie A. Calad、K. A. WoerpelDOI:10.1021/ol063072p日期:2007.3.1Chiral quaternary carbon stereocenters can be established with high diastereoselectivity by a silylene transfer/Ireland-Claisen rearrangement. The utility of this method was demonstrated by application to a synthesis of (+)-5-epi-acetomycin. [reaction: see text]可以通过甲硅烷基转移/爱尔兰-克莱森重排以高非对映选择性建立手性季碳立体中心。该方法的实用性通过应用于合成(+)-5-表霉素。[反应:看文字]
-
Substituent effects on the regioselectivity in fluorination of allylic alcohols with DAST作者:Abdelghani Boukerb、Danielle Grée、Mohammed Laabassi、René GréeDOI:10.1016/s0022-1139(98)00115-8日期:1998.2The regioselectivity of the fluorination of various allylic and β-dienoic alcohols with DAST has been studied. Substituent effects are important in these reactions; a strong preference for the secondary fluorides versus the primary ones is observed, especially in the case of alkyl and aryl substituted derivatives.
-
Palladium(O)-catalyzed allylic substitution with allylic alkoxides as substrates作者:Ivo Starý、Irena G. Stará、Pavel KocˇovskýDOI:10.1016/s0040-4020(01)80774-2日期:1994.1A new method has been developed which allows palladium(O)-catalyzed allylic substitution to occur between allylic alcohols and anionic C-nucleophiles: on reaction with Ph3B, the allylic alkoxide 2 is first converted situ into the more reactive species 3 which then undergoes a Pd(O)-catalyzed reaction with lithio diethyl malonate via the η3-complex 6. Allylic alkoxides can be generated in situ either已经开发出一种新的方法,该方法允许在烯丙基醇和阴离子C-亲核试剂之间发生钯(O)催化的烯丙基取代:与Ph 3 B反应时,烯丙基醇盐2首先被原位转化为反应性更高的物质3,然后该物质3进行Pd(O)催化的与锂代丙二酸二乙酯反应经由所述η 3 -配合物6。烯丙基醇盐可以通过相应的醇(1 → 2,例如用BuLi)去质子化,通过将乙烯基镁卤化物添加到相应的醛中而原位生成(4+ 5 → 2),或通过氢化物还原(DIBAH)将α,β-不饱和酮(31 → 32)还原。整个序列可以一锅法进行,适用于可能难以在纯净状态下处理的敏感烯丙醇。虽然烯丙基醇(7和18)和它们的异构体烯丙基(14和15)得到的混合物的单-和双-烯丙基化产品具有的LiCH(CO 2 ET)2,独家monoallylation已经观察到的仲醇(21,23,和26)。
表征谱图
-
氢谱1HNMR
-
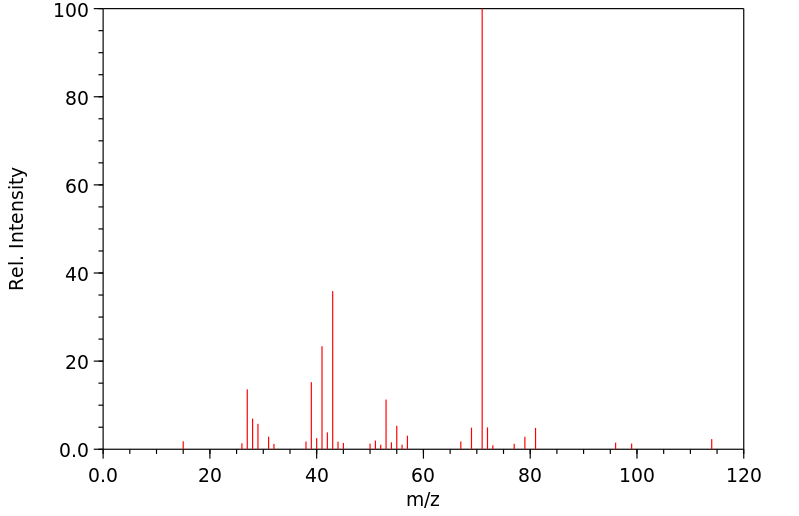
质谱MS
-
碳谱13CNMR
-
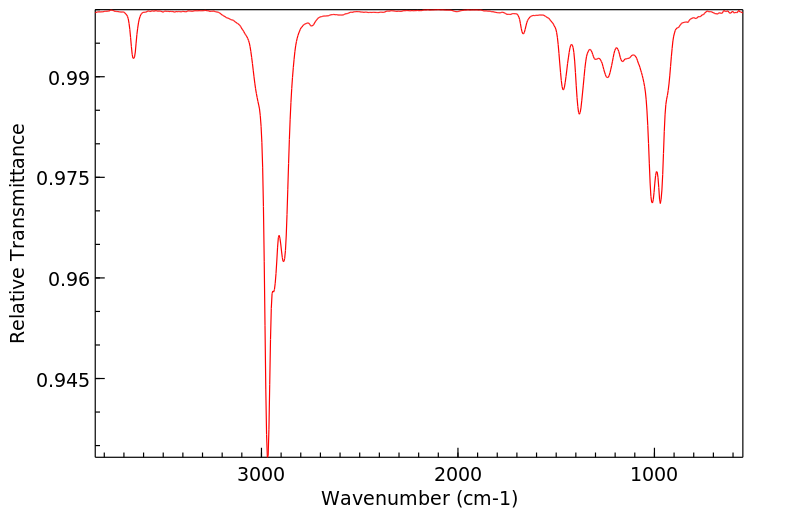
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷