吖啶-N-氧化物 | 10399-73-2
中文名称
吖啶-N-氧化物
中文别名
——
英文名称
acridine N-oxide
英文别名
Acridin-N-oxid;Acridin-N-oxyd;acridine 10-oxide;Acridin-10-oxid;Acridin-10-oxyd;10-oxidoacridin-10-ium
CAS
10399-73-2
化学式
C13H9NO
mdl
——
分子量
195.221
InChiKey
HYGIWTYYBBTQAL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:169 °C
-
沸点:392.3±25.0 °C(Predicted)
-
密度:1.18±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:15
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:25.5
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2933990090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Efficient Deoxygenation of HeteroaromaticN-Oxides with Ammonium Formate as a Catalytic Hydrogen Transfer Agent摘要:在10%的碳载钯存在下,甲酸铵催化转移氢化已显示出在中性介质中对杂环芳香N-氧化物进行温和且优异的去氧化的实用性。DOI:10.1055/s-1989-27349
-
作为产物:描述:参考文献:名称:钌催化的喹啉N-氧化物的脱氧区域选择性C8-H芳基化。摘要:在喹啉上的区域选择性CH官能化引起了增值产品的高度关注。本文中,我们描述了Ru催化喹啉N-氧化物与芳基硼酸酯的脱氧区域选择性C8芳基化的发展。机理研究表明,它以芳基化然后脱氧的串联过程进行,其中发现两个步骤均与钌物种催化。DOI:10.1021/acs.joc.9b01548
文献信息
-
Rh<sup>III</sup>-Catalyzed Dehydrogenative Coupling of Quinoline<i>N</i>-Oxides with Alkenes:<i>N</i>-Oxide as Traceless Directing Group for Remote C-H Activation作者:Ritika Sharma、Rakesh Kumar、Inder Kumar、Upendra SharmaDOI:10.1002/ejoc.201501246日期:2015.12A RhIII-catalyzed dehydrogenative coupling reaction of quinoline N-oxides with alkenes has been developed that provides C-8 olefinated quinoline derivatives by employing a remote C–H activation strategy. Main features of this catalytic method include the use of N-oxide as a traceless directing group, the high selectivity of the reaction for the C-8 position, and the broad scope of possible substrates
-
Co(III)-Catalyzed C–H Amidation of Nitrogen-Containing Heterocycles with Dioxazolones under Mild Conditions作者:Ankit Kumar Dhiman、Ankita Thakur、Inder Kumar、Rakesh Kumar、Upendra SharmaDOI:10.1021/acs.joc.0c01237日期:2020.7.17A cobalt(III)-catalyzed C-8 selective C–H amidation of quinoline N-oxide using dioxazolone as an amidating reagent under mild conditions is disclosed. The reaction proceeds efficiently with excellent functional group compatibility. The utility of the current method is demonstrated by gram scale synthesis of C-8 amide quinoline N-oxide and by converting this amidated product into functionalized quinolines
-
Synthesis of Pyridine<i>N</i>-Oxide-BF<sub>2</sub>CF<sub>3</sub>Complexes and Their Fluorescence Properties作者:Tomoaki Nishida、Aiko Fukazawa、Eriko Yamaguchi、Hiroya Oshima、Shigehiro Yamaguchi、Motomu Kanai、Yoichiro KuninobuDOI:10.1002/asia.201301688日期:2014.4Pyridine N‐oxide–BF2CF3 and –BF2C2F5 complexes and their derivatives were synthesized. Most of the complexes show fluorescence both in solution and in the solid state. By expanding the π‐conjugated skeleton, the color of the fluorescence could be changed dramatically. A fluorophore with a high solvent dependency could also be produced. Since such compounds can be synthesized on a gram scale in high
-
Rh <sup>III</sup> ‐Catalyzed Straightforward Synthesis of Benzophenanthroline and Benzophenanthrolinone Derivatives using Anthranils作者:Aniruddha Biswas、Souradip Sarkar、Rajarshi SamantaDOI:10.1002/chem.201806373日期:——amination/annulation strategy was developed for the synthesis of benzophenanthroline derivatives using quinoline N‐oxides and anthranils. The method was further extended to the synthesis of nitrogen‐containing extended π‐conjugated benzophenanthrolinone derivatives. Late‐stage functionalizations of cinchonidine and cinchophen derivatives and synthesis of a bioactive quinolino‐indole were achieved.
-
Clean protocol for deoxygenation of epoxides to alkenes <i>via</i> catalytic hydrogenation using gold作者:Jhonatan L. Fiorio、Liane M. RossiDOI:10.1039/d0cy01695k日期:——The epoxidation of olefin as a strategy to protect carbon–carbon double bonds is a well-known procedure in organic synthesis, however the reverse reaction, deprotection/deoxygenation of epoxides is much less developed, despite its potential utility for the synthesis of substituted olefins. Here, we disclose a clean protocol for the selective deprotection of epoxides, by combining commercially available烯烃的环氧化是保护碳-碳双键的一种策略,是有机合成中众所周知的方法,然而,尽管逆反应,环氧化物的脱保护/脱氧反应却很少开发,尽管它可以用于合成取代的烯烃。在这里,我们通过结合市售的有机磷配体和金纳米颗粒(Au NP)公开了一种用于环氧化物选择性脱保护的清洁方法。除了成功地用于环氧化物的脱氧中,发现的催化体系还能够使用分子氢作为还原剂选择性还原N-氧化物和亚砜。金NP催化剂与亚磷酸三乙酯P(OEt)3结合与仅金纳米颗粒相比,它的反应性明显更高。该方法不仅是在温和条件下的互补的Au催化还原反应,而且是一种选择性还原各种有价值的分子的有效方法,这些分子在合成上不方便,或者通过替代合成方案或使用经典方法难以获得,过渡金属催化剂。
表征谱图
-
氢谱1HNMR
-
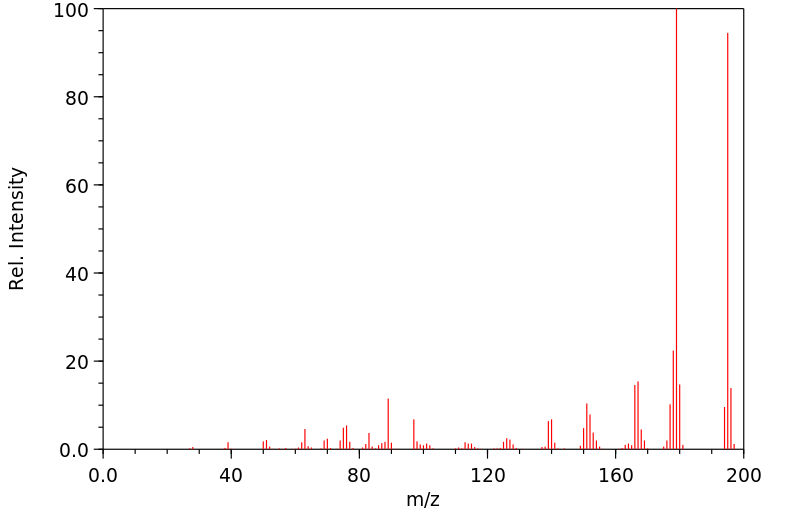
质谱MS
-
碳谱13CNMR
-
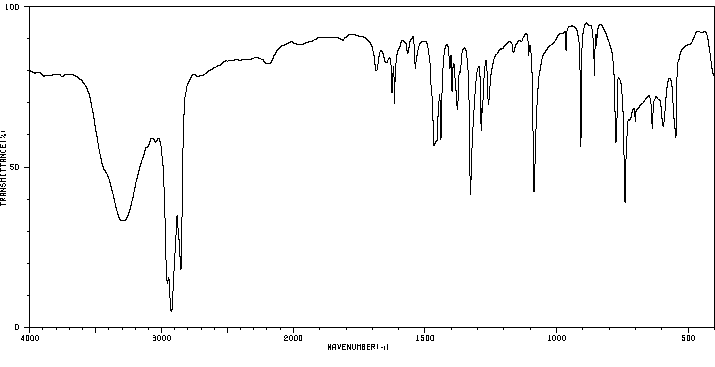
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43