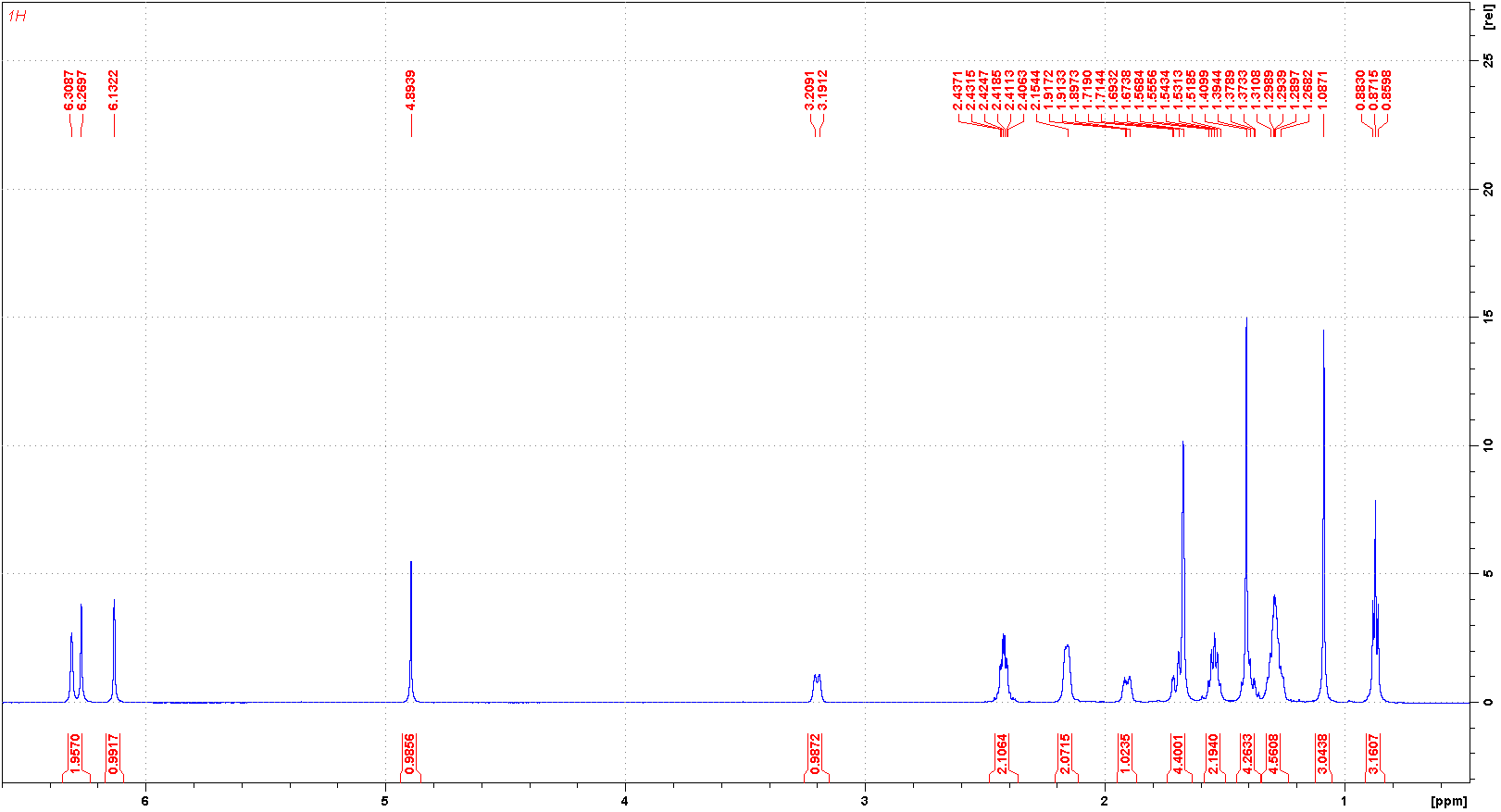
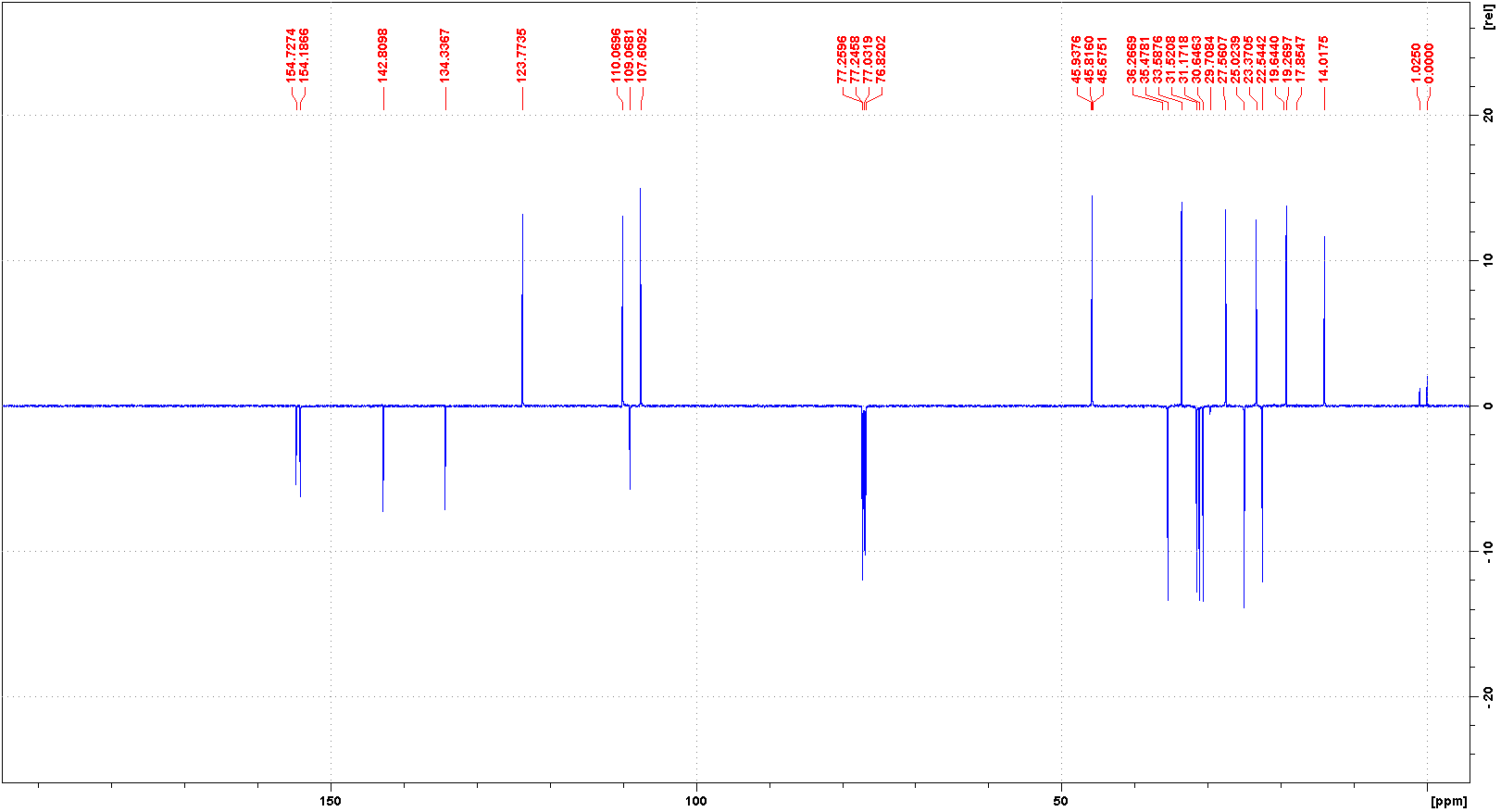
代谢
大麻素(THC)主要在肝脏通过微粒体羟基化和氧化反应进行代谢,这些反应由细胞色素P450酶催化。11-羟基-Δ9-四氢大麻酚(11-OH-THC)是主要的活性代谢物,能够产生心理和行为效应,然后它被代谢成11-nor-9-羧基-Δ9-四氢大麻酚(THC-COOH),这是大麻素的主要非活性代谢物。屈大麻酚及其主要活性代谢物11-OH-Δ9-THC在血浆中的浓度大约相等。口服给药后,母药和代谢物的浓度在大约0.5到4小时达到峰值,并在几天内下降。
THC is primarily metabolized in the liver by microsomal hydroxylation and oxidation reactions catalyzed by Cytochrome P450 enzymes. 11-hydroxy-▵9-tetrahydrocannabinol (11-OH-THC) is the primary active metabolite, capable of producing psychological and behavioural effects, which is then metabolized into 11-nor-9-carboxy-▵ 9-tetrahydrocannabinol (THC-COOH), THC's primary inactive metabolite. Dronabinol and its principal active metabolite, 11-OH-delta-9-THC, are present in approximately equal concentrations in plasma. Concentrations of both parent drug and metabolite peak at approximately 0.5 to 4 hours after oral dosing and decline over several days.
来源:DrugBank