孕甾二醇 | 80-92-2
中文名称
孕甾二醇
中文别名
5β-孕烷-3α,20α-二醇;3-噻吩丙二酸
英文名称
pregnanediol
英文别名
5β-pregnane-3α,20α-diol;(20S)-5β-pregnane-3α,20-diol;3α,20β-Dihydroxy-5β-pregnan;(3R,5R,8R,9S,10S,13S,14S,17S)-17-[(1S)-1-hydroxyethyl]-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol
CAS
80-92-2
化学式
C21H36O2
mdl
——
分子量
320.516
InChiKey
YWYQTGBBEZQBGO-BERLURQNSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:240-242°C
-
比旋光度:D20 +27.4° (c = 0.7 in alc)
-
沸点:399.39°C (rough estimate)
-
密度:1.1500
-
溶解度:氯仿(微溶)、乙醇(微溶)、甲醇(微溶)
-
物理描述:Solid
-
保留指数:2769;2770
计算性质
-
辛醇/水分配系数(LogP):5.2
-
重原子数:23
-
可旋转键数:1
-
环数:4.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2942000000
SDS
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5b-孕甾烷-20alpha-醇-3-酮 20α-Hydroxy-5β-pregnan-3-one 54353-11-6 C21H34O2 318.5 [(1S)-1-[(3R,5R,8R,9S,10S,13S,14S,17S)-3-乙酰氧基-10,13-二甲基-2,3,4,5,6,7,8,9,11,12,14,15,16,17-十四氢-1H-环戊并[a]菲-17-基]乙基]乙酸酯 3α,20αF-diacetoxy-5β-pregnane 1174-69-2 C25H40O4 404.59 孕烷醇酮 PREGNANOLONE 128-20-1 C21H34O2 318.5 还原胆烷醇酮 Etiocholanolone 53-42-9 C19H30O2 290.446 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 [(1S)-1-[(3R,5R,8R,9S,10S,13S,14S,17S)-3-乙酰氧基-10,13-二甲基-2,3,4,5,6,7,8,9,11,12,14,15,16,17-十四氢-1H-环戊并[a]菲-17-基]乙基]乙酸酯 3α,20αF-diacetoxy-5β-pregnane 1174-69-2 C25H40O4 404.59 孕烷醇酮 PREGNANOLONE 128-20-1 C21H34O2 318.5 (2R,4aS,4bS,6aS,7S,8R,10aS,10bR,12aR)-7-氯-4a,6a,8-三甲基-1,2,3,4,4b,5,6,7,8,9,10,10a,10b,11,12,12a-十六氢屈-2-醇 17α-methyl-17aβ-chloro-D-homo-5β-androstane-3α-ol 76571-91-0 C21H35ClO 338.961
反应信息
-
作为反应物:描述:孕甾二醇 在 rabbit 3-hydroxyhexobarbital dehydrogenase (AKR1C29) 、 烟酰胺腺嘌呤双核苷酸磷酸盐 作用下, 以 aq. phosphate buffer 、 乙酸乙酯 为溶剂, 反应 0.5h, 生成 孕烷醇酮参考文献:名称:兔3-羟基己糖巴比妥脱氢酶是一种NADPH优先的还原酶,对酮类固醇,前列腺素D(2)以及其他内源性和异源性羰基化合物具有广泛的底物特异性。摘要:3-羟基己异巴比妥脱氢酶(3HBD)催化将NAD(P)(+)链接的3-羟基己异巴比妥氧化为3-羟基己异巴比妥。该酶被认为是异生物醇和某些羟基类固醇的脱氢酶,但其生理功能仍然未知。我们已经纯化了兔3HBD,分离了其cDNA,并检查了其对辅酶和底物的特异性,反应方向性和组织分布。3HBD是醛酮还原酶(AKR)超家族的成员(AKR1C29),并且在7.4的生理pH值下,NADP(H)优于NAD(H)。在与NADPH相关的还原反应中,3HBD对多种醌,酮和醛(包括3-,17-和20-酮类固醇和前列腺素D(2))显示出广泛的底物特异性,它们被转化为3alpha-,17beta-和20alpha -羟基类固醇和9alpha,11beta-前列腺素F(2),分别。特别是,α-二酮(如isatin和diacetyl)和脂质过氧化衍生的醛(如4-oxo-和4-hydroxy-2-nonenals)是显示低K(m)值(0DOI:10.1016/j.bcp.2013.08.024
-
作为产物:描述:孕烷醇酮 在 rabbit 3-hydroxyhexobarbital dehydrogenase (AKR1C29) 、 还原型辅酶II(NADPH)四钠盐 作用下, 以 aq. phosphate buffer 、 乙酸乙酯 为溶剂, 反应 0.5h, 生成 孕甾二醇参考文献:名称:兔3-羟基己糖巴比妥脱氢酶是一种NADPH优先的还原酶,对酮类固醇,前列腺素D(2)以及其他内源性和异源性羰基化合物具有广泛的底物特异性。摘要:3-羟基己异巴比妥脱氢酶(3HBD)催化将NAD(P)(+)链接的3-羟基己异巴比妥氧化为3-羟基己异巴比妥。该酶被认为是异生物醇和某些羟基类固醇的脱氢酶,但其生理功能仍然未知。我们已经纯化了兔3HBD,分离了其cDNA,并检查了其对辅酶和底物的特异性,反应方向性和组织分布。3HBD是醛酮还原酶(AKR)超家族的成员(AKR1C29),并且在7.4的生理pH值下,NADP(H)优于NAD(H)。在与NADPH相关的还原反应中,3HBD对多种醌,酮和醛(包括3-,17-和20-酮类固醇和前列腺素D(2))显示出广泛的底物特异性,它们被转化为3alpha-,17beta-和20alpha -羟基类固醇和9alpha,11beta-前列腺素F(2),分别。特别是,α-二酮(如isatin和diacetyl)和脂质过氧化衍生的醛(如4-oxo-和4-hydroxy-2-nonenals)是显示低K(m)值(0DOI:10.1016/j.bcp.2013.08.024
文献信息
-
Epimerization of Tertiary Carbon Centers via Reversible Radical Cleavage of Unactivated C(sp<sup>3</sup>)–H Bonds作者:Yaxin Wang、Xiafei Hu、Cristian A. Morales-Rivera、Guo-Xing Li、Xin Huang、Gang He、Peng Liu、Gong ChenDOI:10.1021/jacs.8b05753日期:2018.8.1cleavage of C(sp3)-H bonds can enable racemization or epimerization, offering a valuable tool to edit the stereochemistry of organic compounds. While epimerization reactions operating via cleavage of acidic C(sp3)-H bonds, such as the Cα-H of carbonyl compounds, have been widely used in organic synthesis and enzyme-catalyzed biosynthesis, epimerization of tertiary carbons bearing a nonacidic C(sp3)-H bondC(sp3)-H 键的可逆断裂可以实现外消旋化或差向异构化,为编辑有机化合物的立体化学提供了宝贵的工具。虽然通过裂解酸性 C(sp3)-H 键(例如羰基化合物的 Cα-H)进行的差向异构化反应已广泛用于有机合成和酶催化生物合成,但带有非酸性 C(sp3) 的叔碳的差向异构化-H 键更具挑战性,可用的实用方法很少。在这里,我们报告了第一个合成有用的协议,用于在温和条件下通过未活化的 C(sp3)-H 键与高价碘试剂苯并恶唑叠氮化物和 H2O 的可逆自由基裂解来进行叔碳差向异构化。这些反应对各种环烷烃的未活化 3° CH 键表现出优异的反应性和选择性,并为编辑传统方法难以处理的碳支架的立体化学构型提供了强大的策略。机理研究表明,N3• 作为催化氢原子穿梭的独特能力对于以高效率和选择性可逆地破坏和重组 3° CH 键至关重要。
-
Mass spectra of sterically crowded trialkylsilyl ether derivatives of steroids作者:M.A. Quilliam、J.B. WestmoreDOI:10.1016/0039-128x(77)90012-5日期:1977.5appears to depend upon conformational and stereochemical factors, as well as the position of the parent silyloxy group, RX2SiO, on the steroid skeleton. This particular fragmentation appears to be a powerful diagnostic method for distinguishing between stereoisomers, being especially useful for differentiation between epimers. In addition, the presence of a 17-silyloxy function promotes a characteristic标题化合物的电子轰击质谱具有一些重要特征,这些特征使这些衍生物比广泛研究的三甲基甲硅烷基类似物具有某些优势。碎片的分布明显较少,并且(M-t.Bu)+或(M-i.Pr)+处的丰富离子可作为分子量的指标,对于选择离子监测很有用。从各种前体中,通过提议的多中心过渡态消除HX2SiOH的难易程度似乎取决于构象和立体化学因素,以及母体甲硅烷氧基RX2SiO在类固醇骨架上的位置。该特定片段化似乎是区分立体异构体的有力诊断方法,对区分差向异构体特别有用。此外,17-甲硅烷氧基官能团的存在促进骨骼饱和类固醇中环B的特征性裂解。在双甲硅烷基化的类固醇裂解的各个阶段消除硅烷醇RX2SiOH也是一个重要的过程,但固醇三甲基甲硅烷基醚光谱的其他熟悉特征(尽管通常存在)被大大抑制。
-
[EN] LIPID PRODRUGS OF NEUROSTEROIDS<br/>[FR] PROMÉDICAMENTS LIPIDIQUES DE NEUROSTÉROÏDES申请人:PURETECH LYT INC公开号:WO2021159021A1公开(公告)日:2021-08-12The present invention provides lymphatic system-directing lipid prodrugs, pharmaceutical compositions thereof, methods of producing such prodrugs and compositions, as well as methods of improving the bioavailability or other properties of a therapeutic agent that comprises part of the lipid prodrug. The present invention also provides methods of treating a disease, disorder, or condition such as those disclosed herein, comprising administering to a patient in need thereof a disclosed lipid prodrug or a pharmaceutical composition thereof.本发明提供了淋巴系统导向的脂质前药、其药物组合物、制备此类前药和组合物的方法,以及提高包含在脂质前药中的治疗剂的生物利用度或其他特性的方法。本发明还提供了治疗如本文所述的疾病、紊乱或状况的方法,包括向有需要的患者施用所述的脂质前药或其药物组合物。
-
[EN] INHIBITING HYDROCARBON HYDRATE AGGLOMERATION<br/>[FR] INHIBITION DE L'AGGLOMÉRATION D'HYDRATES D'HYDROCARBURES申请人:COMMW SCIENT IND RES ORG公开号:WO2018218281A1公开(公告)日:2018-12-06A process for inhibiting the formation of gas hydrates in a hydrocarbon fluid comprising adding to the hydrocarbon fluid, a gas hydrate anti-agglomerate which is a biodegradable anti-agglomerant derived from a naturally occurring substance.
-
Isolation and structural elucidation of the degradation products of pregnanediol disulfate obtained by hot acid hydrolysis (Clinical analysis on steroids. XXI).作者:ITSUO YOSHIZAWA、RYOKO OHUCHI、KYOKO NAGATA、SHINJI ITOH、NORIO KAWAHARADOI:10.1248/cpb.30.2474日期:——Besides the main products, 17α-ethyl-17β-methyl-18-nor-5β-androst-13-en-3α-ol (7a), 17α-methyl-D-homo-5β-androstane-3α, 17aβ-diol (8), and 17α-methyl-17aβ-chloro-D-homo-5β-androstan-3α-ol (9), other minor degradation products of pregnanediol 3, 20-disulfate (2) were obtained under reflux in 3 N hydrochloric acid, and were structurally identified by comparison with synthetic specimens. The steroidal mono olefins isolated were 5β-pregn-3-en-20α-ol (10a), 5β-pregn-2-en-20α-ol (11a), 5β-pregn-20-en-3α-ol (12a), and 5β-pregn-17 (20)-en-3α-ol (13a). The Z-isomer of 13a, 5β-pregn-17 (20)-en-3α-ol (14a), was detected by gas chromatography and identified by gas chromatography-mass spectrometric comparison with synthetic compound. Steroidal dienes were obtained as a mixture of Δ2-and Δ3-compounds having partial D-ring structures corresponding to 7a, 12a, and 13a. Although it was a minor product, 5β-pregnane-3α, 20β-diol (16) was also obtained, and its isolation suggests the formation of a C20-carbonium ion in the course of the hydrolysis. The yield of intact steroid, pregnanediol (1), was only 18.4% of the total products obtained.除了主要产品17α-乙基-17β-甲基-18-去氢-5β-雄甾-13-烯-3α-醇(7a)、17α-甲基-D-同雄甾-5β-醇(8)和17α-甲基-17β-氯-D-同雄甾-5β-醇(9),在3 N盐酸回流条件下还获得了孕醇-3,20-二硫酸酯(2)的其他少量降解产物,并通过与合成样品的比较对其结构进行了鉴定。分离得到的类固醇单烯包括5β-孕-3-烯-20α-醇(10a)、5β-孕-2-烯-20α-醇(11a)、5β-孕-20-烯-3α-醇(12a)和5β-孕-17(20)-烯-3α-醇(13a)。以气相色谱法检测到的13a的Z异构体为5β-孕-17(20)-烯-3α-醇(14a),并通过与合成化合物的气相色谱-质谱比较进行了鉴定。分离的类固醇二烯作为混合物包括具有部分D环结构的Δ2和Δ3化合物,对应于7a、12a和13a。虽然它是一个少量产物,5β-孕烷-3α、20β-二醇(16)也被获得,其分离表明在水解过程中形成了C20碳正离子。完整类固醇孕醇(1)的产率仅为获得的总产品的18.4%。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
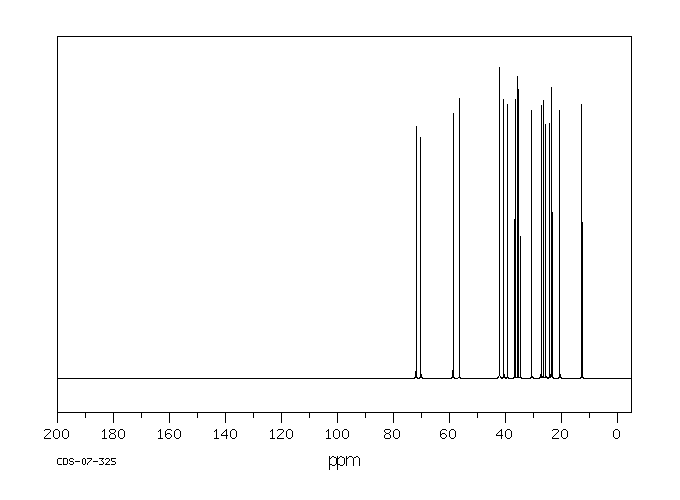
碳谱13CNMR
-
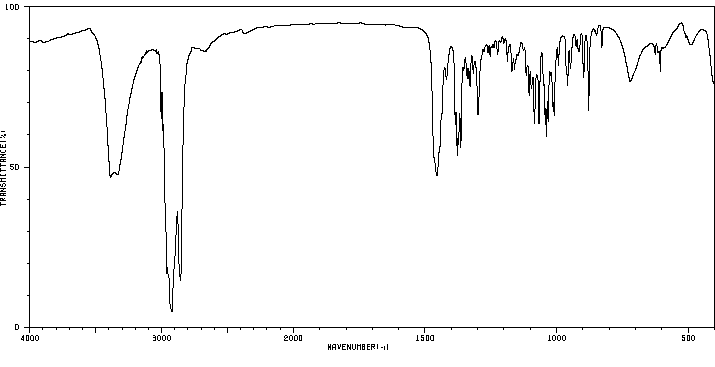
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β)-17,20:20,21-双[亚甲基双(氧基)]孕烷-3-酮
(5α)-2′H-雄甾-2-烯并[3,2-c]吡唑-17-酮
(3β,20S)-4,4,20-三甲基-21-[[[三(异丙基)甲硅烷基]氧基]-孕烷-5-烯-3-醇-d6
(25S)-δ7-大发酸
(20R)-孕烯-4-烯-3,17,20-三醇
(11β,17β)-11-[4-({5-[(4,4,5,5,5-五氟戊基)磺酰基]戊基}氧基)苯基]雌二醇-1,3,5(10)-三烯-3,17-二醇
齐墩果酸衍生物1
黄麻属甙
黄芪皂苷III
黄芪皂苷 II
黄芪甲苷 IV
黄芪甲苷
黄肉楠碱
黄果茄甾醇
黄杨醇碱E
黄姜A
黄夹苷B
黄夹苷
黄夹次甙乙
黄夹次甙乙
黄夹次甙丙
黄体酮环20-(乙烯缩醛)
黄体酮杂质EPL
黄体酮杂质1
黄体酮杂质
黄体酮杂质
黄体酮EP杂质M
黄体酮EP杂质G(RRT≈2.53)
黄体酮EP杂质F
黄体酮6-半琥珀酸酯
黄体酮 17alpha-氢过氧化物
黄体酮 11-半琥珀酸酯
黄体酮
麦角甾醇葡萄糖苷
麦角甾醇氢琥珀酸盐
麦角甾烷-6-酮,2,3-环氧-22,23-二羟基-,(2b,3b,5a,22R,23R,24S)-(9CI)
麦角甾烷-3,6,8,15,16-五唑,28-[[2-O-(2,4-二-O-甲基-b-D-吡喃木糖基)-a-L-呋喃阿拉伯糖基]氧代]-,(3b,5a,6a,15b,16b,24x)-(9CI)
麦角甾烷-26-酸,5,6:24,25-二环氧-14,17,22-三羟基-1-羰基-,d-内酯,(5b,6b,14b,17a,22R,24S,25S)-(9CI)
麦角甾-8-烯-3-醇
麦角甾-8,24(28)-二烯-26-酸,7-羟基-4-甲基-3,11-二羰基-,(4a,5a,7b,25S)-
麦角甾-7,22-二烯-3-酮
麦角甾-7,22-二烯-17-醇-3-酮
麦角甾-5,24-二烯-26-酸,3-(b-D-吡喃葡萄糖氧基)-1,22,27-三羟基-,d-内酯,(1a,3b,22R)-
麦角甾-5,22,25-三烯-3-醇
麦角甾-4,6,8(14),22-四烯-3-酮
麦角甾-1,4-二烯-3-酮,7,24-二(乙酰氧基)-17,22-环氧-16,25-二羟基-,(7a,16b,22R)-(9CI)
麦角固醇
麦冬皂苷D
麦冬皂苷D
麦冬皂苷 B