(E)4-氯-3-甲氧基-2-丁稀酸甲酯 | 110104-60-4
中文名称
(E)4-氯-3-甲氧基-2-丁稀酸甲酯
中文别名
甲酯(E)-4-氯-3-甲氧基-巴豆酸;(E)-4-氯-3-甲氧基-2-丁稀酸甲酯;(E)-4-氯-3-甲氧基-2-丁烯酸甲酯
英文名称
(E)-4-chloro-3-methoxy-but-2-enoic acid methyl ester
英文别名
(E)-methyl 4-chloro-3-methoxy-2-butenoate;methyl (E)-4-chloro-3-methoxy-2-butenoate;methyl (E)-4-chloro-3-methoxybut-2-enoate;methyl 4-chloro-3-methoxy-2-(E)-butenoate;methyl 4-chloro-3-methoxy-2(E)-butenoate;methyl 4-chloro-3-methoxy-2-butenoate
CAS
110104-60-4
化学式
C6H9ClO3
mdl
——
分子量
164.589
InChiKey
JNYMRXDQVPIONI-HWKANZROSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:25 °C
-
沸点:95-97 °C (15.002 mmHg)
-
密度:1.21 g/mL at 25 °C(lit.)
-
闪点:104 °C
-
稳定性/保质期:
在常温常压下保持稳定,但应避免与不相容的材料接触。它可能会与强氧化剂和强碱发生反应。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S45
-
危险类别码:R34
-
海关编码:2918990090
-
危险品运输编号:UN 3261
-
包装等级:III
-
危险类别:8
-
危险性防范说明:P501,P260,P264,P280,P303+P361+P353,P301+P330+P331,P363,P304+P340+P310,P305+P351+P338+P310,P405
-
危险性描述:H314
-
储存条件:密封保存,存放在阴凉干燥的仓库中,并远离腐蚀性物质。
SDS
(E)-4-氯-3-甲氧基-2-丁稀酸甲酯 修改号码:5
模块 1. 化学品
产品名称: Methyl (E)-4-Chloro-3-methoxy-2-butenoate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 1C类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): (E)-4-氯-3-甲氧基-2-丁稀酸甲酯
(E)-4-氯-3-甲氧基-2-丁稀酸甲酯 修改号码:5
模块 3. 成分/组成信息
百分比: >95.0%(GC)
CAS编码: 110104-60-4
俗名: (E)-4-Chloro-3-methoxy-2-butenoic Acid Methyl Ester
分子式: C6H9ClO3
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
(E)-4-氯-3-甲氧基-2-丁稀酸甲酯 修改号码:5
模块 8. 接触控制和个体防护
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 30°C (凝固点)
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
(E)-4-氯-3-甲氧基-2-丁稀酸甲酯 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
UN编号: 1759
正式运输名称: 腐蚀性固体, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Methyl (E)-4-Chloro-3-methoxy-2-butenoate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 1C类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): (E)-4-氯-3-甲氧基-2-丁稀酸甲酯
(E)-4-氯-3-甲氧基-2-丁稀酸甲酯 修改号码:5
模块 3. 成分/组成信息
百分比: >95.0%(GC)
CAS编码: 110104-60-4
俗名: (E)-4-Chloro-3-methoxy-2-butenoic Acid Methyl Ester
分子式: C6H9ClO3
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
(E)-4-氯-3-甲氧基-2-丁稀酸甲酯 修改号码:5
模块 8. 接触控制和个体防护
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 30°C (凝固点)
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氯化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
(E)-4-氯-3-甲氧基-2-丁稀酸甲酯 修改号码:5
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
UN编号: 1759
正式运输名称: 腐蚀性固体, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:(E)4-氯-3-甲氧基-2-丁稀酸甲酯 在 sodium hydroxide 、 氨 作用下, 以 甲醇 、 水 为溶剂, 反应 6.0h, 生成 (1,5-dihydro-4-methoxy-2-oxo-2H-pyrrol-1-yl)acetamide参考文献:名称:用四酸中间体高效合成外消旋4-羟基-2-氧-1-吡咯烷乙酰胺(奥西西坦)摘要:由(E)-4-氯-3-乙氧基丁-2-烯酸乙酯以消旋形式合成标题化合物1a,分五个步骤,总收率为43%。描述了最终产品的几种途径,但是最有效的途径涉及C(5)-未取代的四甲酸5b的氢化。这类化合物的不稳定性要求使用可以在非常酸性的水性条件下运行的催化剂,这种作用已被5%Ru / C催化剂成功填补。DOI:10.1002/hlca.19920750323
-
作为产物:描述:4-氯-3-氧代丁酰氯 在 盐酸 、 sodium hydroxide 、 甲烷磺酸 、 sodium chloride 作用下, 以 氯化亚砜 、 二氯甲烷 、 甲苯 为溶剂, 以80%的产率得到(E)4-氯-3-甲氧基-2-丁稀酸甲酯参考文献:名称:Process for the production of 4-chloro-3-alkoxy-but-2E-enoic acid alkyl摘要:一种从4-氯乙酰乙酸氯通过与二烷基亚磺酸酯反应制备4-氯-3-烷氧基-2E-烯酸烷基酯的工艺。所生产的中间产物被用作药物等结构元素。公开号:US04966989A1
文献信息
-
POLYCYCLIC PYRAZOLINONE DERIVATIVE AND HERBICIDE COMPRISING SAME AS EFFECTIVE COMPONENT THEREOF申请人:SAGAMI CHEMICAL RESEARCH INSTITUTE公开号:US20160024110A1公开(公告)日:2016-01-28Provided are a polycyclic pyrazolinone derivative indicated by general formula (1) (in the formula, R 1 , X 1 , X 2 , X 3 , and Y indicate the definitions provided in the Specification) and a herbicide comprising same as effective component thereof.
-
Novel fused pyrrolocarbazoles申请人:Hudkins L. Robert公开号:US20050137245A1公开(公告)日:2005-06-23The present invention relates generally to selected fused pyrrolocarbazoles, including pharmaceutical compositions thereof and methods of treating diseases therewith. The present invention is also directed to intermediates and processes for making these fused pyrrolocarbazoles.
-
PYRROLIDINONE GLUCOKINASE ACTIVATORS申请人:Berthel Steven Joseph公开号:US20090264445A1公开(公告)日:2009-10-22Provided herein are compounds of the formula (I): as well as pharmaceutically acceptable salts thereof, wherein the substituents are as those disclosed in the specification. These compounds, and the pharmaceutical compositions containing them, are useful for the treatment of metabolic diseases and disorders such as, for example, type II diabetes mellitus.提供的化合物为公式(I): 以及药学上可接受的盐,其中取代基如说明书中所披露。这些化合物以及包含它们的药物组合物,可用于治疗代谢性疾病和障碍,例如,2型糖尿病。
-
A Flexible Approach to (<i>S</i>)-5-Alkyl Tetramic Acid Derivatives: Application to the Asymmetric Synthesis of (+)-Preussin and Protected (3<i>S</i>,4<i>S</i>)-AHPPA作者:Pei-Qiang Huang、Tian-Jun Wu、Yuan-Ping RuanDOI:10.1021/ol035617a日期:2003.11.1text] A flexible asymmetric approach to 5-alkyl tetramic acid derivatives is described, which is based on the use of 9 as the first synthetic equivalent to chiral nonracemic tetramic acid 5-carbanionic synthon 9b. The existence of the carbanion intermediate 9b was proven by trapping with trimethylchlorosilane. Application of the present method to the synthesis of antifungal alkaloid (+)-preussin, as well
-
A Flexible Approach for the Asymmetric Synthesis of N-Protected (<i>R</i>)-5-Alkyl Tetramates and (<i>R</i>)-5-Alkyl Tetramic Acid Derivatives作者:Pei-Qiang Huang、Jun DengDOI:10.1055/s-2003-44968日期:——A flexible two-step asymmetric approach to N-protected (R)-5-alkyl tetramates and (R)-5-alkyl tetramic acid derivatives is described. The method is based on the diastereoselective alkylation of (R)-phenylglycinol derived tetramates 7 and 8, which are the first synthetic equivalents to chiral nonracemic tetramate 5-carbanionic synthons A.
表征谱图
-
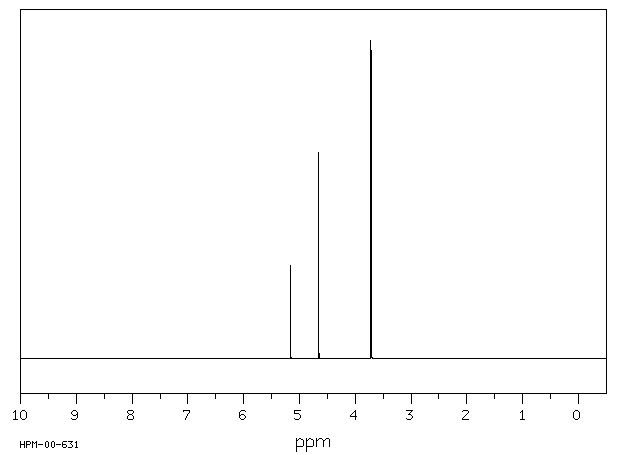
氢谱1HNMR
-
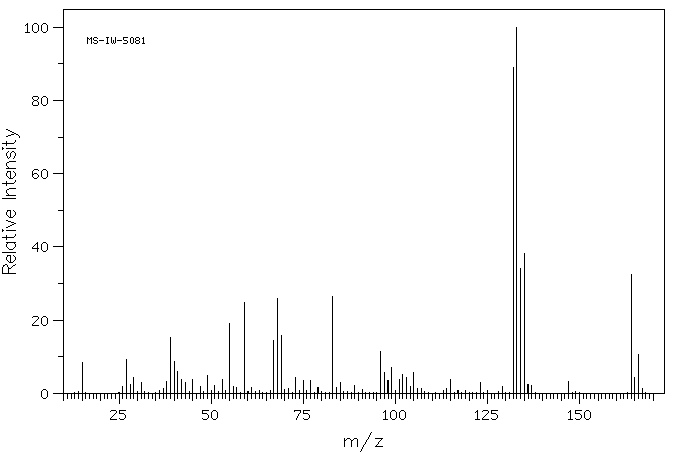
质谱MS
-
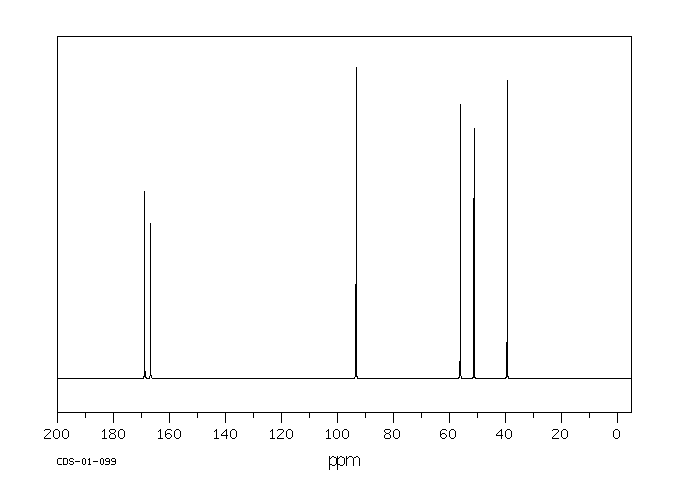
碳谱13CNMR
-
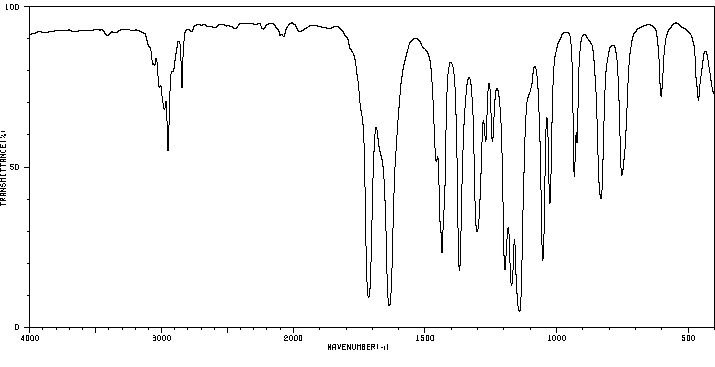
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯