(S)-(-)-甲基丁二酸 | 2174-58-5
中文名称
(S)-(-)-甲基丁二酸
中文别名
S-(-)-2-甲基-1,4-丁二酸;(S)-(-)-甲基琥珀酸;(S)-2-甲基丁二酸
英文名称
L-malic acid
英文别名
(S)-methylsuccinic acid;(S)-2-methylsuccinic acid;methylsuccinic acid;(2S)-2-methylsuccinic acid;(S)-(-)-Methylsuccinic acid;(2S)-2-methylbutanedioic acid
CAS
2174-58-5
化学式
C5H8O4
mdl
MFCD00002659
分子量
132.116
InChiKey
WXUAQHNMJWJLTG-VKHMYHEASA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:110-115 °C(lit.)
-
比旋光度:-8 º (c=5, H2O)
-
沸点:236.5±13.0 °C(Predicted)
-
密度:1.311±0.06 g/cm3(Predicted)
-
物理描述:Solid
-
溶解度:516.5 mg/mL
计算性质
-
辛醇/水分配系数(LogP):-0.2
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:74.6
-
氢给体数:2
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2917190090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:室温且干燥
SDS
模块 1. 化学品
1.1 产品标识符
: (S)-(-)-甲基丁二酸
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
响应
P302 + P352 如皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C5H8O4
分子式
: 132.11 g/mol
分子量
组分 浓度或浓度范围
(S)-(-)-Methylsuccinic acid
-
化学文摘登记号(CAS 2174-58-5
No.)
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
对光线敏感 热敏感。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 110 - 115 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
加热。
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甲基丁二酸 2-methylbutanedioic acid 498-21-5 C5H8O4 132.116 (2S)-甲基丁二酸 4-甲酯 (S)-2-Methyl-succinic acid 4-methyl ester 111266-27-4 C6H10O4 146.143 甲基丁二酸二甲酯 dimethyl methylsuccinate 1604-11-1 C7H12O4 160.17 丙烷-1,1,2-三羧酸 Propantricarbonsaeure 61713-72-2 C6H8O6 176.126 —— 2-hydroxy-2-methylbutane-1,4-dioic acid 597-44-4 C5H8O5 148.116 (S)-4-(叔丁氧基)-2-甲基-4-氧代丁酸 (S)-4-(tert-butoxy)-2-methyl-4-oxobutanoic acid 182007-75-6 C9H16O4 188.224 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (S)-dimethyl 2-methylsuccinate —— C7H12O4 160.17
反应信息
-
作为反应物:描述:(S)-(-)-甲基丁二酸 在 5%-palladium/activated carbon 、 氢气 、 三乙胺 、 N,N-二异丙基乙胺 、 乙酰氯 、 2-溴苯乙酮 、 N,N'-羰基二咪唑 、 三氟乙酸 、 N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate 作用下, 以 四氢呋喃 、 甲醇 、 二氯甲烷 、 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 反应 36.75h, 生成 (3S)-β-phenylalanine-L-valine-(4S)-methylsuccinimide参考文献:名称:Design, Synthesis, and Antibacterial Properties of Dual-Ligand Inhibitors of Acetyl-CoA Carboxylase摘要:There is an urgent demand for the development of new antibiotics due to the increase in drug-resistant pathogenic bacteria. A novel target is the multifunctional enzyme acetyl-CoA carboxylase (ACC), which catalyzes the first committed step in fatty acid synthesis and consists of two enzymes: biotin carboxylase and carboxyltransferase. Covalently attaching known inhibitors against these enzymes with saturated hydrocarbon linkers of different lengths generated dual-ligand inhibitors. Kinetic results revealed that the dual-ligands inhibited the ACC complex in the nanomolar range. Microbiology assays showed that the dual-ligand with a 15-carbon linker did not exhibit any antibacterial activity, while the dual-ligand with a 7-carbon linker displayed broad-spectrum antibacterial activity as well as a decreased susceptibility in the development of bacterial resistance. These results suggest that the properties of the linker are vital for antibacterial activity and show how inhibiting two different enzymes with the same compound increases the overall potency while also impeding the development of resistance.DOI:10.1021/jm501082n
-
作为产物:参考文献:名称:Asymmetric hydrogenation of prochiral olefins catalyzed by rhodium complexes with chiral pyrrolidinodiphosphines. Crucial factors for the effective asymmetric induction摘要:DOI:10.1021/jo01311a036
文献信息
-
Asymmetric Hydrogenation Using Diphenylphosphinite Derivatives of Carbohydrates as the Chiral Ligand of Rhodium Catalysts作者:Mitsuji Yamashita、Manabu Naoi、Hiroyuki Imoto、Tatsuo OshikawaDOI:10.1246/bcsj.62.942日期:1989.3Diphenylphosphinite derivatives of carbohydrates (L-rhamnose, D-mannitol, and cellulose), which have asymmetric centers on the skeletons were synthesized. Asymmetric hydrogenations of prochiral substrates using rhodium catalysts with the prepared chiral phosphinite ligand were carried out to obtain chiral substances in 8–78% optical yield.
-
Inverted Supercritical Carbon Dioxide/Aqueous Biphasic Media for Rhodium-Catalyzed Hydrogenation Reactions作者:Katja Burgemeister、Giancarlo Franciò、Volker H. Gego、Lasse Greiner、Herbert Hugl、Walter LeitnerDOI:10.1002/chem.200601717日期:2007.3.26supercritical carbon dioxide (scCO(2))/aqueous biphasic system has been used as reaction media for Rh-catalysed hydrogenation of polar substrates. Chiral and achiral CO(2)-philic catalysts were efficiently immobilised in scCO(2) as the stationary phase, while the polar substrates and products were contained in water as the mobile phase. Notably, product separation and catalyst recycling were conducted without倒置的超临界二氧化碳(scCO(2))/双相水溶液已被用作Rh催化极性底物加氢的反应介质。手性和非手性的CO(2)-亲和催化剂被有效地固定在scCO(2)中作为固定相,而极性底物和产物则作为流动相包含在水中。值得注意的是,在不使高压釜减压的情况下进行产物分离和催化剂再循环。催化剂相以高转化率和超过85%的产物回收率重复使用了几次。在大多数重复实验中,经过前两个循环后,浸出的铑和磷的损失被发现低于检测极限。通过在顺序优化中使用单纯形算法,通过最少的实验对反应条件进行了优化。在重复的批处理操作中,总周转次数(TTN)高达1600,周转频率(TOF)高达340 h(-1),而ee高达99%。已经研究了所设计的催化系统的范围,并且已经实施了半连续反应装置。手性配体(R,S)-3-H(2)F(6)-BINAPHOS允许衣康酸和-2-乙酰氨基丙烯酸甲酯的高度对映选择性氢化,并在这些反应介质中具有相当高的催化剂稳定性。
-
Asymmetric hydrogenation of prochiral carboxylic acids and functionalized carbonyl compounds catalysed by ruthenium(II)-binap complexes with aryl nitriles (binap = (R)- or (S)-2,2′-bis(diphenylphosphino)-1,1′-binaphthyl)作者:Liming Shao、Kasumi Takeuchi、Makoto Ikemoto、Toshiyasu Kawai、Masamichi Ogasawara、Hiroshi Takeuchi、Hiroyuki Kawano、Masahiko SaburiDOI:10.1016/0022-328x(92)83466-u日期:1992.2Complexes RuCl2(ArCN)2(binap), II (binap = (R)- or (S)-2,2′-bis(diphenylphosphino)- 1,1′-binaphthyl; ArCN = benzonitrile, a; 2-furancarbonitrile, b; pentafluorobenzonitrile, c) were prepared, and their solution properties were investigated by 31P NMR measurements. The catalytic activities and enantioselectivities for IIa–c catalysed hydrogenation of some prochiral acids were very similar to those provided
-
Palladium Nanoparticle–Graphene Catalysts for Asymmetric Hydrogenation作者:Kornél Szőri、Robert Puskás、György Szőllősi、Imre Bertóti、János Szépvölgyi、Mihály BartókDOI:10.1007/s10562-013-1006-6日期:2013.6We report for the first time the application of palladium nanoparticle-graphene (Pd/Gn) catalysts in the asymmetric hydrogenation of aliphatic α,β-unsaturated carboxylic acids using cinchonidine as chiral modifier. Pd/Gns were prepared by deposition–precipitation from the aqueous phase over graphite oxide and subsequent simultaneous reduction of both the support and the metal precursor with NaBH4.
-
[EN] TUBULYSIN DERIVATIVES AND METHODS FOR PREPARING THE SAME<br/>[FR] DÉRIVÉS DE TUBULYSINE ET LEURS PROCÉDÉS DE PRÉPARATION申请人:UNIV GRONINGEN公开号:WO2020022892A1公开(公告)日:2020-01-30The invention relates to novel means and methods for the synthesis of tubulysin and derivatives thereof, which find their use e.g. as cytotoxic agents in targeted drug delivery. Provided is a method for preparing a tubulysin derivative, comprising reacting compounds A, B and C in a 3- component Passerini reaction, wherein compound A is a carboxylic acid according to the general formula (A); wherein compound B is an aldehyde according to the general formula (B); and wherein compound C is an isocyanide according to the general formula (C).
表征谱图
-
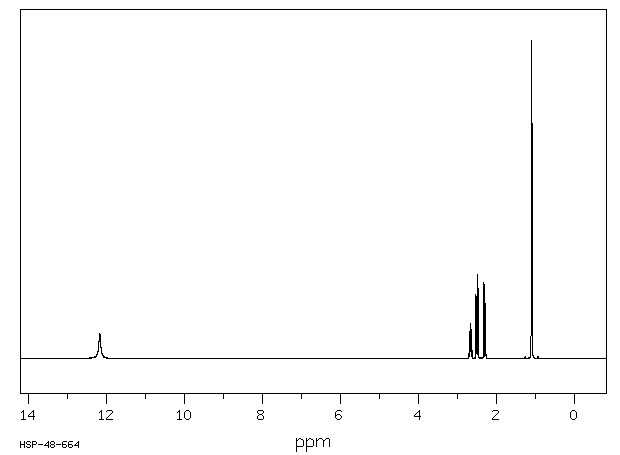
氢谱1HNMR
-
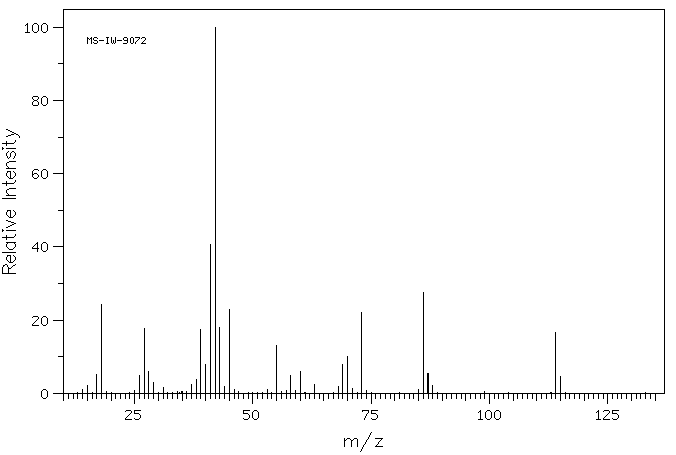
质谱MS
-
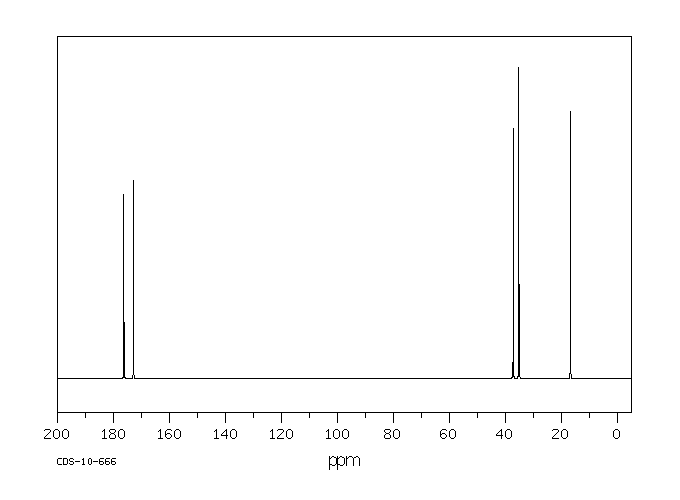
碳谱13CNMR
-
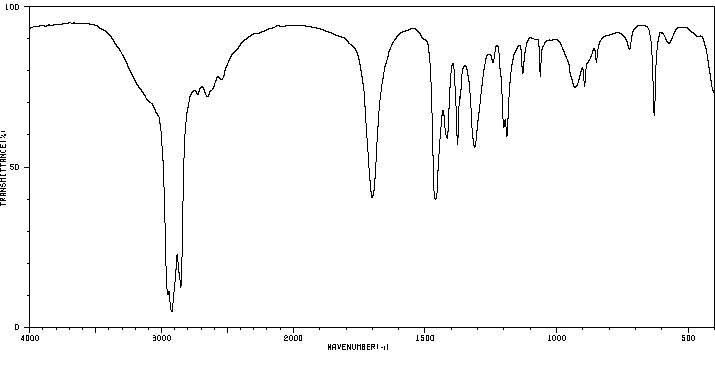
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯