1,2,3,5-四氯苯 | 634-90-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:54.5°C
-
沸点:246°C
-
密度:1.5578 (estimate)
-
闪点:113 °C
-
溶解度:溶于酒精、苯、乙醚和石油醚(Weast,1986)
-
物理描述:1,2,3,5-tetrachlorobenzene appears as white crystals or off-white solid. (NTP, 1992)
-
颜色/状态:Colorless needles
-
蒸汽压力:0.073 mm Hg at 25 °C
-
亨利常数:0.00 atm-m3/mole
-
分解:When heated to decomposition it emits toxic fumes of /hydrogen chloride/.
-
保留指数:1306;1309;1317;1301;1331;1326;1344;1326;1329;1344;1326;1348;1317;1294;1302.7;1312.7
-
稳定性/保质期:
- 稳定性[12]:稳定。
- 禁配物[13]:强氧化剂、强碱。
- 避免接触的条件[14]:受热。
- 聚合危害[15]:不聚合。
- 分解产物[16]:氯化氢。
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
危险品标志:Xn
-
安全说明:S16,S22,S24/25,S36/37,S45,S7
-
危险类别码:R22
-
WGK Germany:3
-
RTECS号:DB9445000
-
海关编码:2903999090
-
危险品运输编号:UN 1230 3/PG 2
-
储存条件:储存注意事项:应将物品存放在阴凉、通风良好的库房中,并远离火源和热源。包装需密封保存,同时与氧化剂、碱类以及食用化学品分开存放,避免混合储存。还需配备相应的消防设备。此外,在储区应准备适当的材料以处理可能的泄漏。
SDS
| 国标编号: | 61659 |
| CAS: | 634-90-2 |
| 中文名称: | 1,2,3,5-四氯苯 |
| 英文名称: | 1,2,3,5-tetrachlorobenzene |
| 别 名: | |
| 分子式: | C 6 H 2 Cl 4 |
| 分子量: | 215.89 |
| 熔 点: | 51℃ 沸点:246℃ |
| 密 度: | |
| 蒸汽压: | 110℃ |
| 溶解性: | 不溶于水,溶于苯、二硫化碳 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色结晶 |
| 危险标记: | 14(有毒品) |
| 用 途: | 用于有机合成 |
2.对环境的影响
该物质对环境可能有危害,建议不要让其进入环境。
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:对眼、上呼吸道、皮肤、粘膜有刺激性。兔吸入本品粉尘,引起红细胞、血红蛋白降低,淋巴细胞增高。重复涂皮引起局部变红,且有全身毒作用。
二、毒理学资料及环境行为
急性毒性: LD501722mg/kg(大鼠经口)
危险特性:遇明火能燃烧。受高热分解产生有毒的腐蚀性烟气。与强氧化剂接触可发生化学反应。
燃烧(分解)产物:一氧化碳、二氧化碳、氯化氢。
3.现场应急监测方法
4.实验室监测方法
气相色谱法《水和废水监测分析方法》(第三版)国家环保局编
气相色谱法《固体废弃物试验分析评价手册》中国环境监测总站等译
5.环境标准
中国(待颁布)饮用水源水中有害物质的最高容许浓度 0.02mg/L
前苏联(1975)水体中有害物质最高允许浓度 0.01mg/L
6.应急处理处置方法
一、泄漏应急处理
隔离泄漏污染区,限制出入。切断火源。建议应急处理人员戴自给式呼吸器,穿一般作业工作服。小量泄漏:用洁净的铲子收集于干燥、洁净、有盖的容器中。大量泄漏:收集回收或运至废物处理场所处置。
二、防护措施
呼吸系统防护:可能接触其粉尘时,应该佩戴自吸过滤式防尘口罩。紧急事态抢救或撤离时,佩戴空气呼吸器。
眼睛防护:戴安全防护眼镜。
身体防护:穿防毒物渗透工作服。
手防护:戴橡胶手套。
其它:工作现场禁止吸烟、进食和饮水。工作毕,彻底清洗。单独存放被毒物污染的衣服。洗后备用。注意个人清洁卫生。
三、急救措施
皮肤接触:脱去被污染的衣着,用肥皂水和清水彻底冲洗皮肤。就医。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。就医。
吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。
食入:饮足量温水,催吐,就医。
灭火剂:泡沫、二氧化碳、砂土。
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 六氯苯 hexachlorobenzene 118-74-1 C6Cl6 284.784 五氯苯 pentachlorobenzene 608-93-5 C6HCl5 250.339 三氯苯 1,2,4-Trichlorobenzene 120-82-1 C6H3Cl3 181.449 1,2,4,5-四氯苯 1,2,4,5-tetrachlorobenzene 95-94-3 C6H2Cl4 215.894 邻二氯苯 1,2-dichloro-benzene 95-50-1 C6H4Cl2 147.004 1,3,5-三氯苯 1,3,5-trichlorobenzene 108-70-3 C6H3Cl3 181.449 1,4-二氯苯 1,4-Dichlorobenzene 106-46-7 C6H4Cl2 147.004 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 六氯苯 hexachlorobenzene 118-74-1 C6Cl6 284.784 五氯苯 pentachlorobenzene 608-93-5 C6HCl5 250.339 三氯苯 1,2,4-Trichlorobenzene 120-82-1 C6H3Cl3 181.449 1,2,4,5-四氯苯 1,2,4,5-tetrachlorobenzene 95-94-3 C6H2Cl4 215.894 三氯苯 1,2,3-trichlorobenzene 87-61-6 C6H3Cl3 181.449 邻二氯苯 1,2-dichloro-benzene 95-50-1 C6H4Cl2 147.004 1,3,5-三氯苯 1,3,5-trichlorobenzene 108-70-3 C6H3Cl3 181.449 1,3-二氯苯 1,3-Dichlorobenzene 541-73-1 C6H4Cl2 147.004 1,4-二氯苯 1,4-Dichlorobenzene 106-46-7 C6H4Cl2 147.004
反应信息
-
作为反应物:描述:参考文献:名称:Photochemistry of polyhaloarenes. 9. Characterization of the radical anion intermediate in the photodehalogenation of polyhalobenzenes摘要:The product-determining intermediate in the photodehalogenation of polyhalobenzenes has been characterized by generating excimers and radical anions within a micellar core and by formation of corresponding radical anions by electron transfer from lithium p,p'-di-tert-butylbiphenyl radical anion (LiDBB). The photodechlorination of pentachlorobenzene (1; 254 nm, CH3CN) produces 1,2,3,5-tetrachloro- (2), 1,2,4,5-tetrachloro- (3), and 1,2,3,4-tetrachlorobenzene (4). The regiochemistry of this reaction is compared with that observed in the photodechlorination of 1 in a micellar solution of hexadecyltrimethylammonium bromide (CTAB) with occupancy numbers (n) principally < 2 and greater-than-or-equal-to 2. Further comparisons with photodechlorination of 1 in a micellar CTAB solution (n < 2) in the presence of triethylamine, as well as photodechlorination in CH3CN in the presence of triethylamine, were used to characterize unencumbered radical anions. The regiochemistries observed in photolytic dehalogenations of 1, 2, 1,2,4-trichlorobenzene, and pentafluorobenzene in the presence of triethylamine are in good agreement with those realized in the radical anion fragmentations induced by electron transfer from LiDBB.DOI:10.1021/jo00011a037
-
作为产物:描述:参考文献:名称:The Partial Additive Chlorination of the Benzene Ring. III. Pentachlorccyclohexene and Hexachlorocyclohexene摘要:DOI:10.1021/ja01634a012
文献信息
-
The reactions of unactivated aryl halides with sodium methoxide in HMPA作者:L. Testaferri、M. Tiecco、M. Tingoli、D. Chianelli、M. MontanucciDOI:10.1016/s0040-4020(01)97647-1日期:——Sodium methoxide reacts with dichlorobenzenes in HMPA to give the chloroanisoles as a result of a SNAr process. Excess MeONa then effects the demethylation of the ethers to give the chlorophenols via an SN2 reaction. With tri- and tetrachlorobenzenes the initially formed chloroanisoles can be dealkylated to chlorophenols or can suffer further substitution to give the chlorodimethoxybenzenes; these
-
Photoreactions of Polyhalobenzenes in Benzene. Formation of Terphenyls作者:Masahiro Nakada、Chihiro Miura、Hideaki Nishiyama、Futoshi Higashi、Toru Mori、Minoru Hirota、Tetsuo IshiiDOI:10.1246/bcsj.62.3122日期:1989.10Polychlorobenzenes, polybromobenzenes, and polychloropolybromobenzenes (C6H6−nXn) were photolyzed in benzene solutions; the products were analyzed by the GC/MS method. Both dehalogenation and phenylation were shown to take place competitively, producing (poly)halobenzenes bearing one less halogen atom (C6H7−nXn−1) and (poly)halobiphenyls (C6H6−nXn−1–C6H5) as the major products. Besides these products
-
Reactions of some fluoroaromatics with the ethoxide anion作者:Joanne H. James、Michael E. Peach、Charles R. WilliamsDOI:10.1016/s0022-1139(00)80901-x日期:1985.1The reactions of sodium ethoxide in ethanol with various fluoroaromatics, C6F6−nHn, C6F5−nHnNO2, C6F5X (X = CF3, C6F5, COCH3, CH2Br), C6Cl6 and mH2C6Cl4 have been studied. Partial substitution of the aromatic halogen was observed. The new products have been characterized by elemental analysis, NMR (H−1 and F−19), infrared and mass spectroscopy.
-
Halogenated volatiles from the fungus <i>Geniculosporium</i> and the actinomycete <i>Streptomyces chartreusis</i>作者:Tao Wang、Patrick Rabe、Christian A Citron、Jeroen S DickschatDOI:10.3762/bjoc.9.311日期:——
Two unidentified chlorinated volatiles
X andY were detected in headspace extracts of the fungusGeniculosporium . Their mass spectra pointed to the structures of a chlorodimethoxybenzene forX and a dichlorodimethoxybenzene forY . The mass spectra of some constitutional isomers forX andY were included in our databases and proved to be very similar, thus preventing a full structural assignment. For unambiguous structure elucidation all possible constitutional isomers forX andY were obtained by synthesis or from commercial suppliers. Comparison of mass spectra and GC retention times rigorously established the structures of the two chlorinated volatiles. Chlorinated volatiles are not very widespread, but brominated or even iodinated volatiles are even more rare. Surprisingly, headspace extracts fromStreptomyces chartreusis contained methyl 2-iodobenzoate, a new natural product that adds to the small family of iodinated natural products.在真菌Geniculosporium的头空间提取物中检测到了两种未经鉴定的氯化挥发性化合物X和Y。它们的质谱指向X为氯二甲氧基苯,Y为二氯二甲氧基苯的结构。X和Y的一些构造异构体的质谱已包含在我们的数据库中,证明它们非常相似,因此无法完全确定结构。为了明确结构,我们通过合成或从商业供应商处获得了X和Y的所有可能的构造异构体。质谱和气相色谱保留时间的比较严格确定了这两种氯化挥发性化合物的结构。氯化挥发性化合物并不是很常见,但溴化或甚至碘化的挥发性化合物更为罕见。令人惊讶的是,从Streptomyces chartreusis的头空间提取物中发现了甲基2-碘苯甲酸酯,这是一种新的天然产物,丰富了碘化天然产物家族。 -
Ozone-Mediated Reaction of Polychlorobenzenes and Some Related Halogeno Compounds with Nitrogen Dioxide: A Novel Non-Acid Methodology for the Selective Mononitration of Moderately Deactivated Aromatic Systems作者:Hitomi Suzuki、Tadashi Mori、Koichi MaedaDOI:10.1055/s-1994-25586日期:——In the presence of ozone and preferably methanesulfonic acid as catalyst, polychlorobenzenes undergo selective mononitration with nitrogen dioxide at low temperatures, giving the corresponding polychloronitrobenzenes, in most cases in nearly quantitative yields.
表征谱图
-
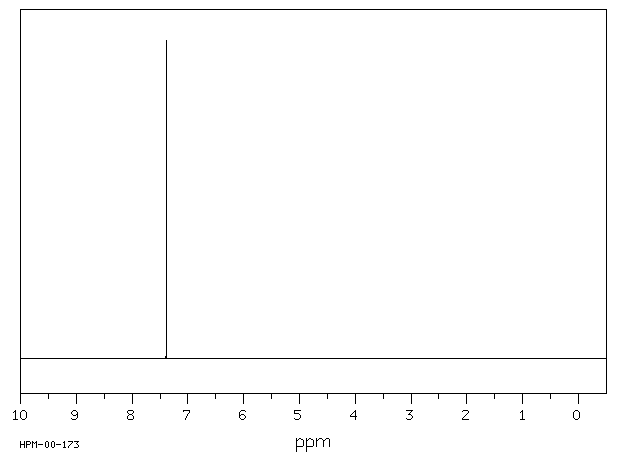
氢谱1HNMR
-
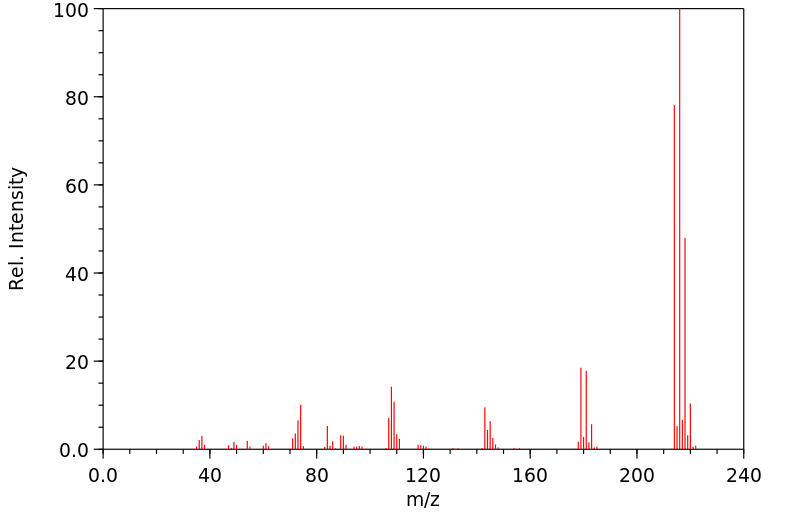
质谱MS
-
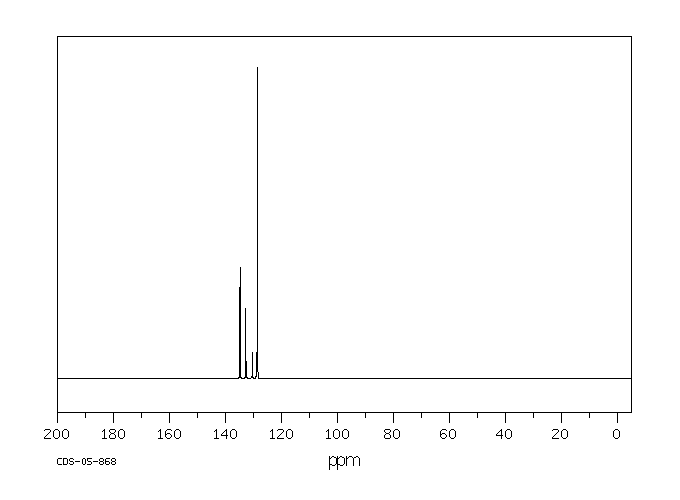
碳谱13CNMR
-
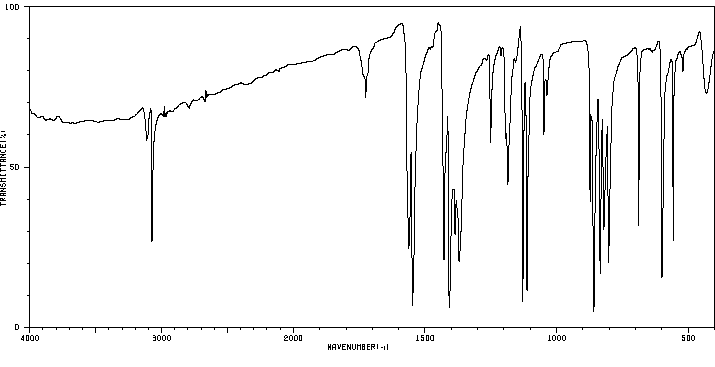
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息