1,3-戊二醇 | 149-31-5
中文名称
1,3-戊二醇
中文别名
——
英文名称
2-methyl-1,3-pentanediol
英文别名
2-methylpentane-1,3-diol;2-Methylpentan-1,3-diol
CAS
149-31-5
化学式
C6H14O2
mdl
MFCD01740992
分子量
118.176
InChiKey
SPXWGAHNKXLXAP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-30°C
-
沸点:214-215℃ (700 Torr)
-
密度:0.9737 g/cm3 (22 ºC)
-
保留指数:1005
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2905399090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:de Montmollin; Martenet, Helvetica Chimica Acta, 1929, vol. 12, p. 608摘要:DOI:
-
作为产物:描述:3-hydroxyl-2-methylpentanal 在 sodium tetrahydroborate 作用下, 以 甲醇 、 水 为溶剂, 反应 1.0h, 以41 mg的产率得到1,3-戊二醇参考文献:名称:脯氨酸表面活性剂组合的有机催化剂,可用于醛的高度非对映体和对映体选择性水性直接羟醛缩合反应。摘要:DOI:10.1002/anie.200601156
文献信息
-
[EN] RENEWABLE FURAN BASED AMINE CURING AGENTS FOR EPOXY THERMOSET<br/>[FR] AGENTS DE DURCISSEMENT AMINE À BASE DE FURANE RENOUVELABLE POUR PLASTIQUE ÉPOXYDIQUE THERMODURCISSABLE申请人:PALMESE GIUSEPPE R公开号:WO2019040389A1公开(公告)日:2019-02-28The present invention relates novel furan based amine cross-linkers with improved thermomechanical and water barrier properties. The novelty of this invention is the use of aromatic, and hydrophobic aliphatic aldehydes to bridge two furfuryl amines, which yields a diamine or tetra amines with a significantly enhanced hydrophobic character. These diamine cross-linkers exhibit enhanced water barrier properties and thermomechanical properties when cured with both commercial and synthetic epoxies.
-
The Direct, Enantioselective, One-Pot, Three-Component, Cross-Mannich Reaction of Aldehydes: The Reason for the Higher Reactivity of Aldimineversus Aldehyde in Proline-Mediated Mannich and Aldol Reactions作者:Yujiro Hayashi、Tatsuya Urushima、Mitsuru Shoji、Tadafumi Uchimaru、Isamu ShiinaDOI:10.1002/adsc.200505190日期:2005.10In the proline-mediated Mannich and aldol reactions of propanal as a nucleophile, the aldimine prepared from benzaldehyde and p-anisidine is about 7 times more reactive than the corresponding aldehyde, benzaldehyde, as an electrophile. This higher reactivity of aldimine over aldehyde is attributed to the carboxylic acid of proline protonating the basic nitrogen atom of the aldimine more effectively
-
Asymmetric cross- and self-aldol reactions of aldehydes in water with a polystyrene-supported triazolylproline organocatalyst作者:Patricia Llanes、Sonia Sayalero、Carles Rodríguez-Escrich、Miquel A. PericàsDOI:10.1039/c6gc00792a日期:——A polystyrene-immobilized triazolylproline has been prepared by a bottom-up approach involving co-polymerization with full regiocontrol. The resulting PS resin swells in water and has been applied to the enantioselective cross-aldol...
-
Substrate-Dependent Stereospecificity of Tyl-KR1: An Isolated Polyketide Synthase Ketoreductase Domain from<i>Streptomyces fradiae</i>作者:Matthias Häckh、Michael Müller、Steffen LüdekeDOI:10.1002/chem.201300554日期:2013.7.1configuration of the Tyl‐KR1 reduction products was determined by using vibrational circular dichroism (VCD) spectroscopy combined with quantum chemical calculations. The conversion of only one of the tested substrates, 2‐methyl‐3‐oxovaleric acid N‐acetylcysteamine thioester, afforded the expected anti‐(2R,3R) configuration of the α‐methyl‐β‐hydroxyl ester product, representing the stereochemistry observed for酶促反应的立体特异性取决于底物及其对映异构体与活性位点结合的方式。这些绑定模式无法轻易预测。我们通过分析五种不同的酮酸酯底物还原的立体化学结果,研究了来自链霉菌的酪酮聚酮化合物合酶的酮还原酶结构域Tyl-KR1的立体特异性和立体选择性。通过使用振动圆二色性(VCD)光谱结合量子化学计算来确定Tyl-KR1还原产物的绝对构型。仅一种被测底物2甲基3氧代戊酸N乙酰半胱胺硫酯的转化提供了预期的抗(2 R,3 R)α-甲基-β-羟基酯产物的构型,代表观察到的生理学聚酮化合物酪酸内酯的立体化学。对于所有其他根据酯的类型和/或链长(C 4代替C 5)进行修饰的底物,获得了对映体相反的构型(anti-(2 S,3 S)),对映体和非对映选择性。两个立体中心的倒置表明完全不同的结合模式仅通过对底物结构的微小修改即可调用。
-
Optical Element for Correcting Color Blindness申请人:Harding Brett T.公开号:US20130032758A1公开(公告)日:2013-02-07Described herein are devices, compositions, and methods for improving color discernment.本文描述了用于改善色彩辨识的设备、组合物和方法。
表征谱图
-
氢谱1HNMR
-
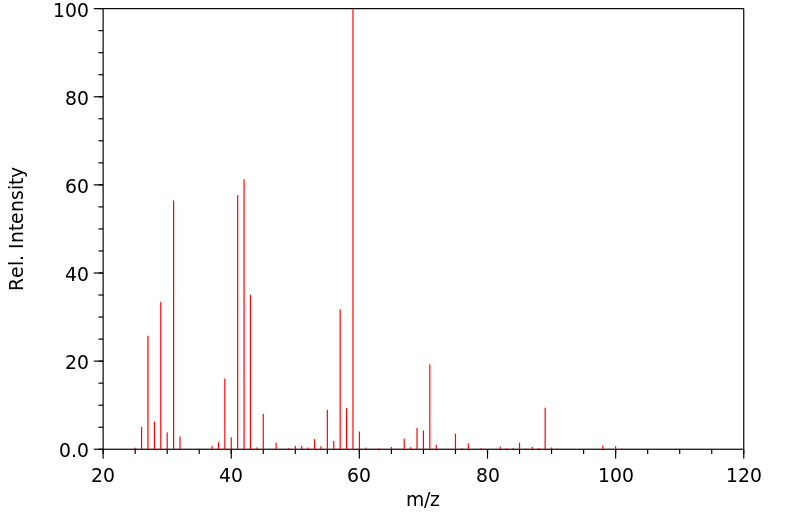
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷