1,4-二乙酰氧基萘 | 5697-00-7
中文名称
1,4-二乙酰氧基萘
中文别名
——
英文名称
1,4-diacetoxynaphthalene
英文别名
1,4-Diacetoxy-naphthalin;naphthalene-1,4-diyl diacetate;(4-acetyloxynaphthalen-1-yl) acetate
CAS
5697-00-7
化学式
C14H12O4
mdl
——
分子量
244.247
InChiKey
SFAVMNCWIUOBCX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:18
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
储存条件:请将药品存放在避光、阴凉干燥的地方,并密封保存。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-乙酰氧基-4-羟基萘 4-acetoxy-1-naphthol 70662-30-5 C12H10O3 202.21 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-乙酰氧基-4-羟基萘 4-acetoxy-1-naphthol 70662-30-5 C12H10O3 202.21 4-异丙氧基萘-1-醇 4-isopropyloxynaphth-1-ol 41426-37-3 C13H14O2 202.253 —— (4-Phenylmethoxynaphthalen-1-yl) acetate 352200-67-0 C19H16O3 292.334 —— (R)-(-)-1-benzyloxy-4-(oxiranylmethoxy)naphthalene 352200-69-2 C20H18O3 306.361 —— (S)-(+)-1-benzyloxy-4-(oxiranylmethoxy)naphthalene 352200-70-5 C20H18O3 306.361 —— 1-Benzyloxy-4-hydroxynaphthalene 73661-06-0 C17H14O2 250.297
反应信息
-
作为反应物:描述:1,4-二乙酰氧基萘 在 (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride 、 sodium tetrahydroborate 、 双氧水 、 氧气 、 potassium acetate 、 potassium carbonate 、 sodium hydroxide 作用下, 以 甲醇 、 乙醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 27.33h, 生成 1,4-萘醌参考文献:名称:H 2 O 2介导的硼酸酯探针对氧化还原循环分子的自催化脱保护的指数分子扩增摘要:在本文中,描述了一种基于H 2 O 2介导的硼酸酯探针脱保护为氧化还原循环化合物的新分子自催化反应方案,在O 2和还原剂或酶的存在下产生指数信号增益。为此,设计了围绕萘醌/萘氢醌氧化还原活性核构建的新型化学传感探针,并被自消旋的硼酸酯保护基团掩盖。使用这些探针,可以通过使用UV / Visible或荧光光学检测或通过电化学监测获得具有特征性滞后和指数相的典型自催化动力学迹线。浓度低到0.5μ的检测米ħ 2 ö在相对较短的时间内(<1小时)即可获得2和0.5 n m的萘醌衍生物。通过与自动催化相关的两个交叉活化的催化环的动力学分析,确定了控制自动催化反应网络的关键参数,间接表明分析性能目前受到硼酸盐探针缓慢的非特异性自保护作用的限制。总的来说,目前的结果证明了这种新的指数分子扩增策略的潜力,由于其通用的性质和模块性,该方法对于与涉及H 2 O 2的各种生物测定方法的耦合是很有前途的。 或氧化DOI:10.1002/chem.201900627
-
作为产物:参考文献:名称:氧取代萘的光氧化摘要:已经研究了氧取代的萘与单线态氧(1 O 2)的反应,并且首次分离了不稳定的过氧化物,并在–78°C下进行了表征。紫外光谱的低温动力学表明,与1,4-二甲基萘相比,烷氧基和甲硅烷氧基取代基显着提高了光氧合速率,而酰氧基取代的并苯对1 O 2呈惰性。反应性与我们通过密度泛函理论计算确定的HOMO能量和自由活化能很好地相关。分离出的过氧化物的不稳定性是由于它们即使在–20°C下在释放1时也会与相应的萘发生非常快速的逆反应。O 2,使其在非常温和的条件下成为该反应物种的上等来源。最终,合成了碳水化合物取代的萘,该萘可与1 O 2可逆反应,并可能在未来的工作中用于对映选择性氧化。DOI:10.1002/poc.3734
文献信息
-
Regioselective Hydrolysis of Diacetoxynaphthalenes Catalyzed by Pseudomonas sp. Lipase in an Organic Solvent作者:Pierangela Ciuffreda、Silvana Casati、Enzo SantanielloDOI:10.1016/s0040-4020(99)01000-5日期:2000.1Depending on the relative positions of the acetyl groups in the aromatic rings, the Pseudomonas sp. lipase-catalyzed hydrolysis of diacetoxynaphthalenes in tert-butylmethyl ether proceeds regioselectively to afford the corresponding monoacetates.
-
Bismuth Triflate-Catalyzed Fries Rearrangement of Aryl Acetates作者:Thierry Ollevier、Valerie Desyroy、Muhammad Asim、Marie-Christine BrochuDOI:10.1055/s-2004-836025日期:——Bismuth triflate was found to be an efficient catalyst in the Fries rearrangement of phenyl or 1-naphthyl acetates. Both reactions proceeded smoothly with a catalytic amount of bismuth triflate (10 mol%) to afford the corresponding hydroxyaryl ketone in moderate to good yields in most cases.
-
Synthetic Utility and Mechanistic Implications of the Fries Rearrangement of Hydroquinone Diesters in Boron Trifluoride Complexes作者:Jessica L. Boyer、Jodie E. Krum、Michael C. Myers、Aleem N. Fazal、Carl T. WigalDOI:10.1021/jo000412q日期:2000.7.1trifluoride etherate complexes results in acetylhydroquinone derivatives. This procedure represents a one-step synthesis of acetylhydroquinone derivatives, important building blocks for a variety of synthetic applications.
-
Stereospecific synthesis of highly substituted novel carbasugar as carbonic anhydrase inhibitors: decahydronaphthalene-1,2,3,4,5,6,7-heptol作者:Latif Kelebekli、Neslihan Balcı、Ertan ŞahinDOI:10.1016/j.tet.2014.05.101日期:2014.8Decahydronaphthalene-1,2,3,4,5,6,7-heptol, a new polycyclitol, was synthesized starting from p-benzoquinone. An endo selective Diels-Alder cycloaddition between p-benzoquinone and 1-acetoxybutadiene followed by stereoselective reduction with NaBH4/CeCl3·7H2O led to the formation of an allylic cis-diol. The formed diol was converted into its acetate with Ac2O/pyridine, in a transformation that required
-
Antifungal Activity of 1,4-Dialkoxynaphthalen-2-Acyl Imidazolium Salts by Inducing Apoptosis of Pathogenic Candida spp.作者:Jisue Lee、Jae-Goo Kim、Haena Lee、Tae Hoon Lee、Ki-Young Kim、Hakwon KimDOI:10.3390/pharmaceutics13030312日期:——pathogenic fungus that can cause candidiasis. The emergence of resistant Candida strains and the toxicity of antifungal agents have encouraged the development of new classes of potent antifungal agents. Novel naphthalen-2-acyl imidazolium salts (NAIMSs), especially 1,4-dialkoxy-NAIMS from 1,4-dihydroxynaphthalene, were prepared and evaluated for antifungal activity. Those derivatives showed prominent anti-Candida尽管念珠菌属。常见于人体皮肤上,也是一种机会性致病真菌,可引起念珠菌病。耐药念珠菌菌株的出现和抗真菌剂的毒性促进了新型强效抗真菌剂的开发。制备了新型萘-2-酰基咪唑鎓盐 (NAIMS),特别是来自 1,4-二羟基萘的 1,4-二烷氧基-NAIMS,并评估了其抗真菌活性。根据微量稀释抗真菌药敏试验,这些衍生物表现出显着的抗念珠菌活性,24 小时内的最低抑菌浓度 (MIC) 为 3.125 至 6.26 μg/mL。在测试的化合物中,NAIMS 7c显示出最强的抗真菌活性,其 MIC 值为 3.125 μg/mL,而咪康唑对念珠菌的 MIC 值为 12.5 μg/mL,更重要的是,对耳念珠菌的 MIC 值>100 μg/mL。 NAIMS 7c处理后,白色念珠菌中活性氧 (ROS) 的产生增加,JC-1 染色显示线粒体膜电位损失。紫外线 (UV) 吸收材料释放的增加表明 NAIMS 7c可能会导致细胞破坏。
表征谱图
-
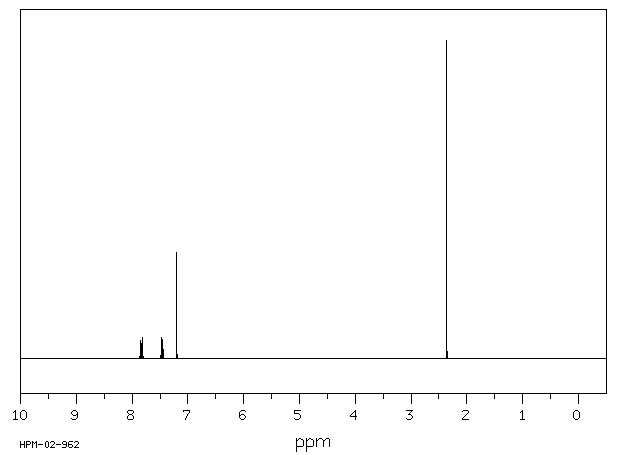
氢谱1HNMR
-
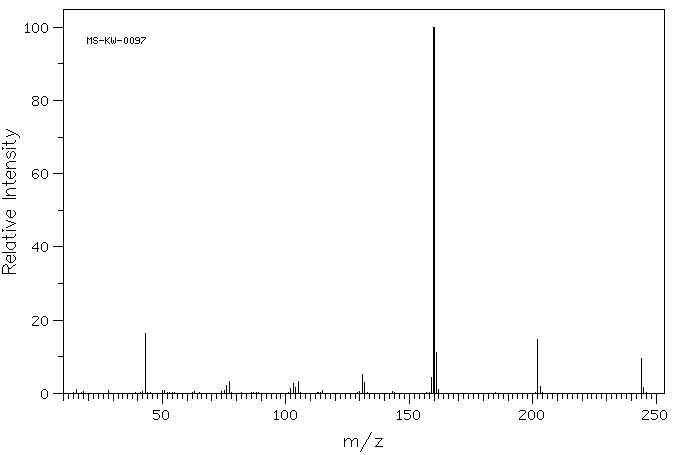
质谱MS
-
碳谱13CNMR
-
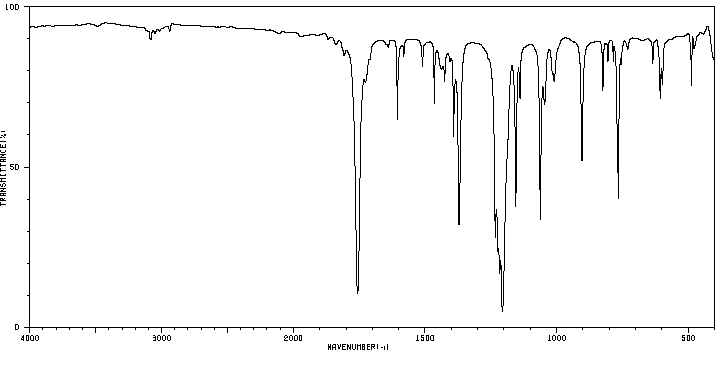
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮