1-戊基-1-环己烯 | 15232-85-6
中文名称
1-戊基-1-环己烯
中文别名
——
英文名称
1-pentyl-1-cyclohexene
英文别名
1-penty-1-cyclohexene;1-pentyl-cyclohexene;1-pentylcyclohexene;1-Pentyl-cyclohexen;1-Pentyl-cyclohexen-(1);1-Amyl-cyclohex-1-en;Cyclohexene, 1-pentyl-
CAS
15232-85-6
化学式
C11H20
mdl
——
分子量
152.28
InChiKey
IOTQTOFPEVQVPG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:203.6±7.0 °C(Predicted)
-
密度:0.823±0.06 g/cm3(Predicted)
-
保留指数:1135
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:11
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.82
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:用重铬酸吡啶鎓氧化烯烃-碘配合物来制备碘代醇和环氧化物的简便方法摘要:用I 2活化的三取代的烯烃被重铬酸吡啶鎓转变为碘醇和环氧化物。该转化显示以区域特异性和立体特异性方式进行。而且,经过类似处理的一些天然存在的多烯选择性地仅提供末端碘醇。后者通过新的方便的氧化铝负载反应转化为相应的环氧化物。DOI:10.1016/s0040-4020(01)88685-3
-
作为产物:描述:参考文献:名称:The Preparation of Some Mono- and Dialkylcyclohexanes摘要:DOI:10.1021/ja01335a047
文献信息
-
Terminal‐Selective Functionalization of Alkyl Chains by Regioconvergent Cross‐Coupling作者:Stéphanie Dupuy、Ke‐Feng Zhang、Anne‐Sophie Goutierre、Olivier BaudoinDOI:10.1002/anie.201608535日期:2016.11.14zinc bromides and regioconvergent Negishi coupling with aryl or alkenyl triflates. The use of a suitable phosphine ligand favoring Pd migration enabled the selective formation of the linear cross‐coupling product. Subsequently, mixtures of secondary alkyl bromides were prepared from linear alkanes by standard bromination, and regioconvergent cross‐coupling then provided access to the corresponding linear
-
<i>p</i>-Toluenesulfonic Acid Adsorbed on Silica Gel: An Efficient Dehydrating Agent of Alcohols作者:Franco D'Onofrio、Arrigo ScettriDOI:10.1055/s-1985-31463日期:——Secondary and tertiary alcohols are efficiently dehydrated by reaction with p-toluenesulfonic acid supported on silica gel. In particular, the procedure allows the direct conversion of 3-hydroxy-steroids into Î2-olefins or Î3,5-dienes, without passing through the mesylate or tosylate esters.
-
Aliphatic Friedel-Crafts reactions. Part VI. Preparation of βγ-unsaturated ketones by the acetylation of substituted cyclohexenes作者:J. K. Groves、N. JonesDOI:10.1039/j39680002215日期:——Reaction of 1-alkylcyclohexenes with zinc chloride–acetic anhydride produces 6-acetyl-1-alkylcyclohexenes in good yields, together with small amounts of the isomeric 1-acetyl-2-alkylidenecyclohexanes. Hydrogenation of of the unstaurated ketones readily affords 1-acetyl-2-alkylcyclohexanes.
-
β- and γ-Disubstituted Olefins: Substrates for Copper-Catalyzed Asymmetric Allylic Substitution作者:Caroline A. Falciola、Karine Tissot-Croset、Hugo Reyneri、Alexandre AlexakisDOI:10.1002/adsc.200800086日期:2008.5.5The copper-catalyzed asymmetric allylic alkylation has shown through many examples that it is a powerful means to generate stereogenic centers with mono β- and γ-substituted olefinic substrates. However, little has been reported about more substituted olefinic patterns, such as β-disubstituted allylic electrophiles. In this paper, we show that a simple procedure using easily accessible Grignard reagents
-
β-Disubstituted Allylic Chlorides: Substrates for the Cu-Catalyzed Asymmetric SN2′ Reaction作者:Caroline A. Falciola、Karine Tissot-Croset、Alexandre AlexakisDOI:10.1002/anie.200601855日期:2006.9.11
表征谱图
-
氢谱1HNMR
-
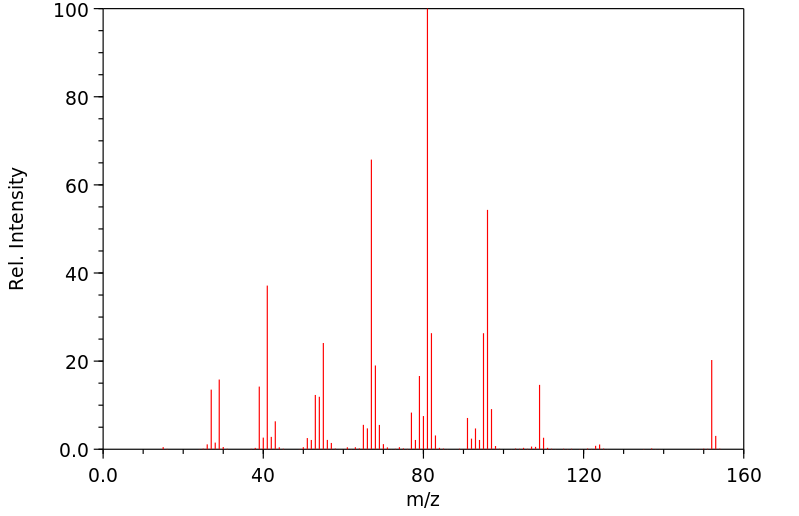
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-