1-甲基-2-哌啶甲酸乙酯 | 30727-18-5
中文名称
1-甲基-2-哌啶甲酸乙酯
中文别名
1-甲基六氢吡啶酸乙酯;甲基哌啶-2-羧酸乙酯;N-甲基-2-哌啶甲酸乙酯;哌啶-2-羧酸甲脂;N-甲基六氢吡啶酸乙酯;1-甲基-2-哌啶甲酸甲;N-甲基-2-哌啶甲酸甲酯
英文名称
ethyl 1-methylpiperidine-2-carboxylate
英文别名
ethyl 1-methylpipecolinate
CAS
30727-18-5
化学式
C9H17NO2
mdl
MFCD00006492
分子量
171.239
InChiKey
ZICBNHLULHJETL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:92-96 °C11 mm Hg(lit.)
-
密度:0.975 g/mL at 25 °C(lit.)
-
闪点:165 °F
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.888
-
拓扑面积:29.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:C
-
安全说明:S24/25,S26,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:3
-
海关编码:2933399090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-哌啶甲酸乙酯 ethyl pipecolate 15862-72-3 C8H15NO2 157.213 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-哌啶甲酸乙酯 ethyl pipecolate 15862-72-3 C8H15NO2 157.213 1-甲基哌啶-2-羧酸 1-methylpiperidine-2-carboxylic acid 7730-87-2 C7H13NO2 143.186 N-Boc-2-哌啶甲酸乙酯 ethyl N-Boc-pipecolinate 362703-48-8 C13H23NO4 257.33
反应信息
-
作为反应物:描述:1-甲基-2-哌啶甲酸乙酯 在 sodium tetrahydroborate 、 正丁基锂 作用下, 以 乙醇 为溶剂, 反应 0.5h, 生成 2-Benzotriazol-1-yl-1-(1-methyl-piperidin-2-yl)-2-p-tolyl-ethanol参考文献:名称:Benzotriazole-Mediated Stereoselective Olefination of Carboxylic Esters: Transformation of α-Amino Acid Esters into Chiral Allylamines摘要:Diastereoselective trans-olefinations of carboxylic esters 3a-h have been accomplished using benzylic or allylic benzotriazole derivatives la-e to prepare alpha-(benzotriazol-1-yl) ketones 4a-i, for the subsequent reduction of 4a-i, and finally for low-valent titanium-effected dehydroxybenzotriazolylation.(1) N-Protected alpha-amino acid esters 9a-c and 15 thus give allylamines 13a-e and 19 with virtually full retention of chirality. Mechanistic aspects of the dehydroxybenzotriazolylation are discussed.DOI:10.1021/jo971546f
-
作为产物:描述:参考文献:名称:Lukes et al., Collection of Czechoslovak Chemical Communications, 1957, vol. 22, p. 286,288, 289摘要:DOI:
文献信息
-
[EN] PRODRUGS OF MYELOPEROXIDASE INHIBITORS<br/>[FR] PROMÉDICAMENTS D'INHIBITEURS DE LA MYÉLOPEROXYDASE申请人:BIOHAVEN THERAPEUTICS LTD公开号:WO2021072140A1公开(公告)日:2021-04-15Disclosed are prodrugs of myeloperoxidase (MPO) inhibitors, methods of treating MPO related disorders, e.g., multiple system atrophy, amyotrophic lateral sclerosis, and Huntingtons disease, and methods of neuroprotection, which include administering to a patient in need thereof the prodrugs, pharmaceutical compositions including the prodrugs, and kits including the pharmaceutical compositions and instructions for use.
-
The ring opening of 3,4-dichloro-1,2,5-thiadiazole with metal amides. A new synthesis of 3,4-disubstituted-1,2,5-thiadiazoles作者:Alain Merschaert、Pascal Boquel、Hugo Gorissen、Jean-Pierre Van Hoeck、Alfio Borghese、Luc Antoine、Vincent Mancuso、Anne Mockel、Michel VanmarsenilleDOI:10.1016/j.tetlet.2006.09.102日期:2006.11We have developed a new synthesis of 3,4-disubstituted-1,2,5-thiadiazoles. The methodology is based on the ring opening of readily available 3,4-dichloro-1,2,5-thiadiazole with metal amides to afford a stable synthon, which is then transformed into the 3,4-disubstituted-1,2,5-thiadiazole derivatives via two consecutive reactions with O-, S-, N- or C-nucleophiles.我们开发了3,4-二取代-1,2,5-噻二唑的新合成方法。该方法基于容易获得的3,4-二氯-1,2,5-噻二唑与金属酰胺的开环以提供稳定的合成子,然后将其转化为3,4-二取代的1,2,5 -噻二唑衍生物通过与O-,S-,N-或C-亲核试剂的两个连续反应而形成。
-
Mechanism of elimination of amino acid derivatives in the gas phase. Pyrolysis kinetics of ethyl picolinate, ethyl 1-methylpipecolinate and picolinic acid作者:Jennifer Lafont、Adolfo Ensuncho、Rosa M. Domínguez、Alexandra Rotinov、Armando Herize、Jairo Quijano、Gabriel ChuchaniDOI:10.1002/poc.568日期:2003.1decarboxylation process. The pyrolysis kinetics of picolinic acid, which is an intermediate of ethyl picolinate elimination, showed a dramatic fast decomposition into pyridine and CO2 gas. The decarboxylation process of α-amino or α-nitrogen substituents of carboxylic acids differs from the decarbonylation elimination of several known α-substituted carboxylic acids in the gas phase. Copyright © 2002 John在静态反应系统中,在180.8–419.4°C的温度范围和15-86 Torr的压力范围(1 Torr = 133.3 Pa)下,确定了气相消除标题化合物的动力学。反应是均相的和单分子的,并且服从一阶速率定律。观察到的速率系数由以下Arrhenius方程表示:对于吡啶甲酸乙酯,log [ k 1(s -1)] =(11.30±0.24)-(180.9±3.0)kJ mol -1(2.303 RT)-1, 1-甲基哌嗪酸乙酯,log [ k 1(s -1)] =(13.36±0.31)-(209.5±3.9)kJ mol -1(2.303 RT)-1对于吡啶甲酸,log [ k 1(s -1)] =(12.05±0.10)-(135.7±0.9)kJ mol -1(2.303 RT)-1。这项工作的数据与文献报道的数据一起,证实了先前的考虑,即氨基酸或羧酸的α-氮取代基会经历非常快速的脱羧过
-
Methyl‐Selective α‐Oxygenation of Tertiary Amines to Formamides by Employing Copper/Moderately Hindered Nitroxyl Radical (DMN‐AZADO or 1‐Me‐AZADO)作者:Satoru Nakai、Takafumi Yatabe、Kosuke Suzuki、Yusuke Sasano、Yoshiharu Iwabuchi、Jun‐ya Hasegawa、Noritaka Mizuno、Kazuya YamaguchiDOI:10.1002/anie.201909005日期:2019.11.11α-oxygenation of tertiary amines by employing a Cu/nitroxyl radical catalyst system. The use of moderately hindered nitroxyl radicals, such as 1,5-dimethyl-9-azanoradamantane N-oxyl (DMN-AZADO) and 1-methyl-2-azaadamanane N-oxyl (1-Me-AZADO), was very important to promote the oxygenation effectively mainly because these N-oxyls have longer life-times than less hindered N-oxyls. Various types of tertiary N-methylamines叔胺的甲基选择性α-加氧是合成甲酰胺同时保留胺底物骨架的极具吸引力的方法。因此,开发能够使用分子氧(O 2)作为末端氧化剂来促进在N-甲基位置上的区域选择性α-氧化的有效催化剂是重要的课题。在这项研究中,我们成功地通过使用Cu / nitroxyl自由基催化剂系统开发了一种高度区域选择性和高效的叔胺好氧甲基选择性α-加氧方法。使用中等受阻的硝酰基自由基,例如1,5-二甲基-9-金刚烷金刚烷N-氧基(DMN-AZADO)和1-甲基-2-氮杂金刚烷N-氧基(1-Me-AZADO)对于有效地促进氧合作用,主要是因为这些N-羟基比受阻较少的N-羟基具有更长的寿命。将各种类型的叔N-甲胺选择性地转化为相应的甲酰胺。还根据实验证据以及DFT计算讨论了合理的反应机理。该催化剂体系的高区域选择性源自胺-N-氧基相互作用的空间限制。
-
Late-Stage <i>N</i>-Me Selective Arylation of Trialkylamines Enabled by Ni/Photoredox Dual Catalysis作者:Yangyang Shen、Tomislav RovisDOI:10.1021/jacs.1c08157日期:2021.10.13The diversity and wide availability of trialkylamines render them ideal sources for rapid construction of complex amine architectures. Herein, we report that a nickel/photoredox dual catalysis strategy affects site-selective α-arylation of various trialkylamines. Our catalytic system shows exclusive N-Me selectivity with a wide range of trialkylamines under mild conditions, even in the context of late-stage
表征谱图
-
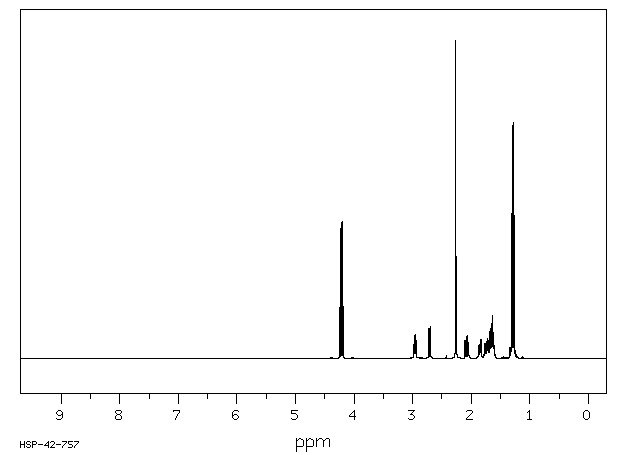
氢谱1HNMR
-
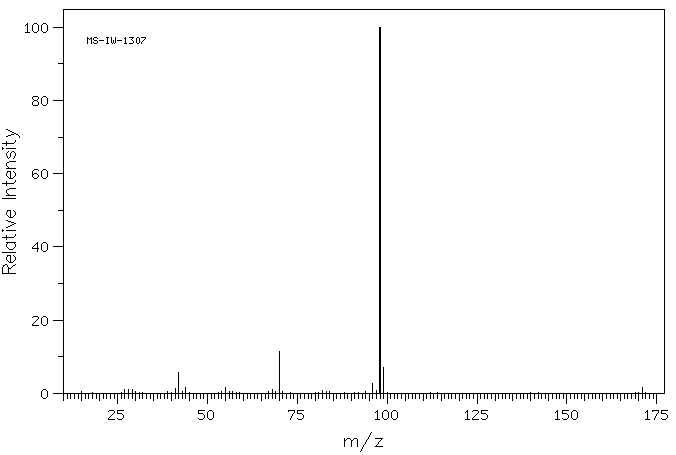
质谱MS
-
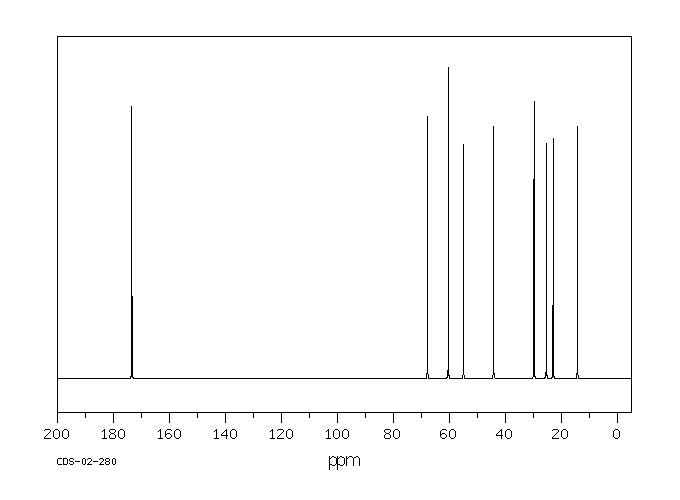
碳谱13CNMR
-
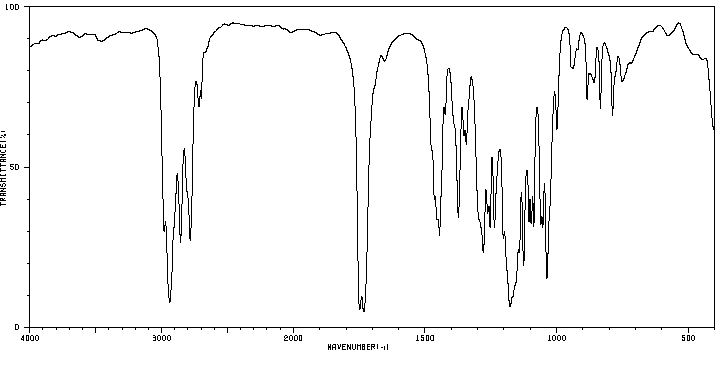
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸