2,3,4-三甲基-5-己烯-3-醇 | 28638-29-1
中文名称
2,3,4-三甲基-5-己烯-3-醇
中文别名
——
英文名称
2,3,4-trimethyl-5-hexen-3-ol
英文别名
CH2CHCH(CH3)C(CH3)(i-C3H7)OH;2,3,4-trimethyl-hex-5-en-3-ol;2,3,4-trimethylhex-5-en-3-ol
CAS
28638-29-1
化学式
C9H18O
mdl
MFCD00060916
分子量
142.241
InChiKey
MXUVULIBGSLKGS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:10
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.777
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905290000
SDS
反应信息
-
作为反应物:描述:2,3,4-三甲基-5-己烯-3-醇 、 三正丁基甲氧基锡 以 not given 为溶剂, 生成参考文献:名称:Preparation of n-Bu(in3)SnCH(in2)CHCH(CH3) and n-Bu2(Cl)SnCH2CHCH(CH3) by elimination reactions of alkoxides of the type n-Bu2(X)SnOC(CH3)(i-C3H7)(CH3)CHCH2 (X = n-Bu and Cl)摘要:DOI:10.1016/s0020-1693(00)94655-3
-
作为产物:描述:参考文献:名称:Miginiac,P., Bulletin de la Societe Chimique de France, 1970, p. 1077 - 1083摘要:DOI:
文献信息
-
Reversible crotylstannation of carbonyl compounds. Crotylstannation ability of Bu3-nClnSnCH2CHCHCH3 (n = 0, 1, 2) compounds towards acetone and benzaldehyde and 13C NMR characterization作者:Alessandro Gambaro、Daniele Marton、Valerio Peruzzo、Giuseppe TagliaviniDOI:10.1016/s0022-328x(00)84584-2日期:1981.1synthesized by means of transalkoxylation between Bu3-nCInSn(OMe) compounds and threo/erithro-2,3,4-trimethyl-5-hexen-3-ol. Under the conditions used the elimination occurs stereospecifically and with complete allylic rearrangement. The ability of the organostannoxy compounds to yield crotyl butylchlorotins via elimination increases in the order, Bu3Sn—O—C < Bu2ClSn—O—C < BuCI2Sn—O—C. In the addition巴豆锡烷基化反应:已发现是可逆的。化合物反式/顺式Bu 3- n Cl n SnCH 2 CH = CHCH 3(n = 0,1,2)是通过有机锡氧基化合物,Bu 3 - n Cl n Sn-OC(Me )的消除反应制备的。)(i-Pr)CH(Me)CH = CH2,它是通过Bu 3- n CI n Sn(OMe)化合物与苏/二甲苯基之间的烷氧基化反应合成的-2,3,4-三甲基-5-己烯-3-醇。在所使用的条件下,消除是立体定向的,并且具有完整的烯丙基重排。有机锡氧基化合物通过消除而产生巴豆基丁基氯锡的能力按以下顺序增加,即Bu 3 Sn-OC = <Bu 2 ClSn-OC = <BuCl 2 Sn-OC =。在加成反应中,反应性增加的顺序是Bu 3 SnCrot
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: Sn: Org.Comp.12, 1.4.1.1.1.5.2.1.1, page 38 - 56作者:DOI:——日期:——
-
Hydrated σ-bonded organometallic cations in organic synthesis作者:Donatella Furlani、Daniele Marton、Giuseppe Tagliavini、Michele ZordanDOI:10.1016/0022-328x(88)89088-0日期:1988.3
-
Peruzzo, V.; Tagliavini, G., Inorganica Chimica Acta作者:Peruzzo, V.、Tagliavini, G.DOI:——日期:——
-
GAMBARO A.; MARTON D.; PERUZZO V.; TAGALIAVINI G., J. ORGANOMETAL. CHEM., 1981, 204, NO 2, 191-196作者:GAMBARO A.、 MARTON D.、 PERUZZO V.、 TAGALIAVINI G.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
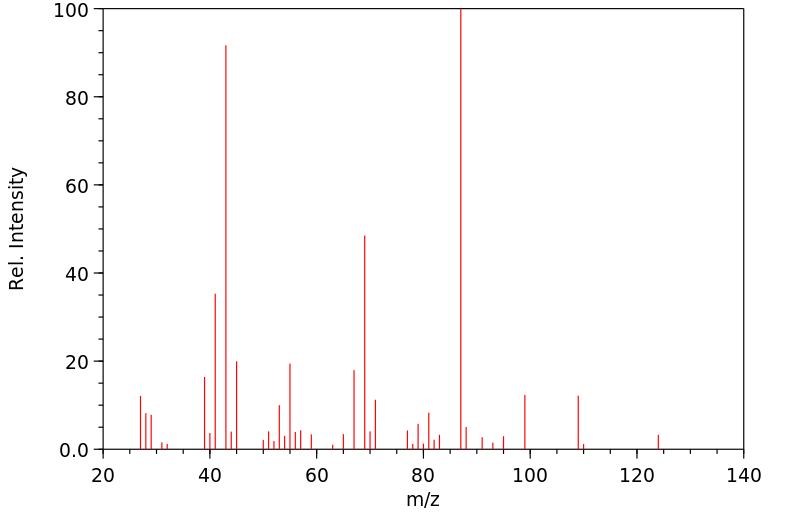
质谱MS
-
碳谱13CNMR
-
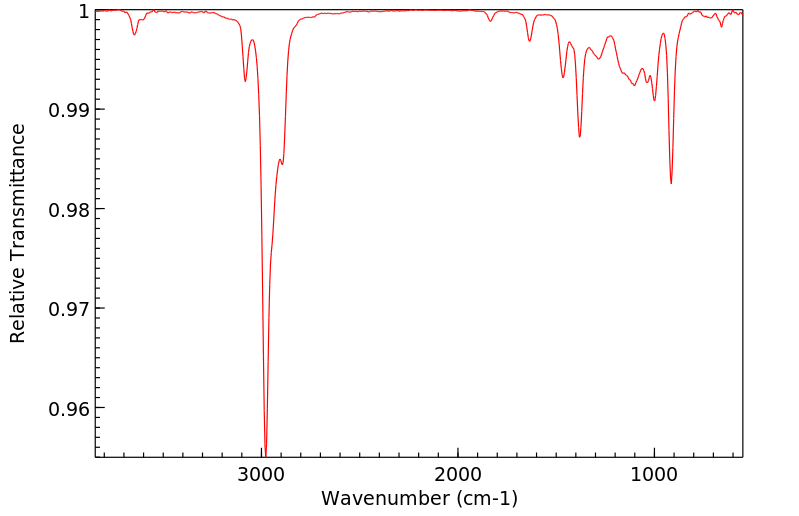
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷