2,4,6-三氨基嘧啶 | 1004-38-2
中文名称
2,4,6-三氨基嘧啶
中文别名
三氨基嘧啶
英文名称
2,4,6-triamino pyrimidine
英文别名
pyrimidine-2,4,6-triamine;TAP;2,4,6-Triaminopyrimidine
CAS
1004-38-2
化学式
C4H7N5
mdl
MFCD00006100
分子量
125.133
InChiKey
JTTIOYHBNXDJOD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:249-251 °C (lit.)
-
沸点:222.58°C (rough estimate)
-
密度:1.2938 (rough estimate)
-
溶解度:DMSO(少量溶解)、甲醇(少量溶解)
-
LogP:-1.95 at 25℃
-
稳定性/保质期:
本品具有刺激作用。若不慎接触眼睛,请立即用大量清水冲洗,并及时就医。操作时,建议佩戴适当的防护用品。
计算性质
-
辛醇/水分配系数(LogP):-0.8
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:104
-
氢给体数:3
-
氢受体数:5
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S36,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2933599090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将容器密封保存,并存放在阴凉、干燥的地方。
SDS
| Name: | 2 4 6-Triaminopyrimidine 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1004-38-2 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1004-38-2 | 2,4,6-Triaminopyrimidine, 97% | 97% | 213-720-7 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. Get medical aid if cough or other symptoms appear.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower.
Exposure Limits CAS# 1004-38-2: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear a chemical apron.
Respirators:
A NIOSH/MSHA approved air purifying dust or mist respirator or European Standard EN 149.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: slightly beige
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 249 - 251 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: slightly soluble
Specific Gravity/Density:
Molecular Formula: C4H7N5
Molecular Weight: 125.13
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, carbon dioxide.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1004-38-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,4,6-Triaminopyrimidine, 97% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 1004-38-2: No information available.
Canada
CAS# 1004-38-2 is listed on Canada's DSL List.
CAS# 1004-38-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1004-38-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-氯-嘧啶-2,4,6-三胺 2,4,6-Triamino-5-chlorpyrimidin 24867-23-0 C4H6ClN5 159.578 2,4,6-三氨基-5-嘧啶甲醛 5-formyl-2,4,6-triaminopyrimidine 88075-69-8 C5H7N5O 153.143 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-亚硝基-2,4,6-三氨基嘧啶 5-nitroso-2,4,6-triaminopyrimidine 1006-23-1 C4H6N6O 154.131 2,4,6-三氨基-5-嘧啶甲醛 5-formyl-2,4,6-triaminopyrimidine 88075-69-8 C5H7N5O 153.143 2-羟基-4,6-二氨基嘧啶 4,6-diamino-2-hydroxypyrimidine 31458-45-4 C4H6N4O 126.118
反应信息
-
作为反应物:描述:参考文献:名称:咪唑并吡啶-和嘌呤-硫代乙酰胺衍生物:核苷酸焦磷酸酶/磷酸二酯酶1(NPP1)的强抑制剂。摘要:核苷酸焦磷酸酶/磷酸二酯酶1(NPP1)属于胞外核苷酸酶家族,可控制细胞外核苷酸,核苷和(di)磷酸盐的水平。为了研究具有药物样特性的NPP1强效和选择性抑制剂的(病理)生理作用。因此,使用比色测定法以对硝基苯基5'-胸苷单磷酸酯(p -Nph-5'-TMP)作为人工底物,筛选化合物库中的NPP1抑制剂。这导致发现2-(3 H-咪唑并[4,5 - b ]吡啶-2-基硫基)-N-(3,4-二甲氧基苯基)乙酰胺(5a)为具有K i的命中化合物。值为217 nM。随后的结构-活性关系研究导致了嘌呤和咪唑并[4,5- b ]吡啶类似物的开发,用p -Nph-5'-进行测定具有高抑制力(K i值分别为5.00 nM和29.6 nM)。以TMP为底物。出乎意料的是,与ATP作为底物相比,测试时这些化合物的效力明显较低,K i值在低微摩尔范围内。对原型抑制剂的抑制机理进行了研究,发现该抑制剂与两种底物都具有竞争性。DOI:10.1021/jm501434y
-
作为产物:描述:5-氯-嘧啶-2,4,6-三胺 在 palladium on activated charcoal 、 sodium acetate 、 铂 作用下, 75.0 ℃ 、5.88 MPa 条件下, 生成 2,4,6-三氨基嘧啶参考文献:名称:Chloroaminopyrimidines摘要:DOI:10.1021/ja01165a504
-
作为试剂:描述:5-(4-Dihexadecylamino-benzylidene)-pyrimidine-2,4,6-trione 在 2,4,6-三氨基嘧啶 、 水 作用下, 生成 巴比妥酸 、 4-(N,N-dihexadecylamino)benzaldehyde参考文献:名称:Ahuja, Ramesh; Caruso, Pier-Lorenzo; Moebius, Dietmar, Angewandte Chemie, 1993, vol. 105, # 7, p. 1082 - 1085摘要:DOI:
文献信息
-
Azo pigment and pigment dispersion申请人:FUJIFILM Corporation公开号:EP2039725A2公开(公告)日:2009-03-25An azo pigment represented by the following formula (1) or a tautomer thereof: wherein R1 represents an aliphatic group having 1 to 8 carbon atoms in total, an aryl group having 6 to 12 carbon atoms in total or a heterocyclic group having 2 to 12 carbon atoms in total; R2 represents a hydrogen atom or an aliphatic group having 1 to 8 carbon atoms in total; R3 represents a hydrogen atom, an aliphatic group having 1 to 4 carbon atoms in total, an aliphatic amino group having 1 to 8 carbon atoms in total, or an amino group; R4 and R5 each independently represent an aliphatic amino group having 1 to 8 carbon atoms in total, or an amino group; R6 represents a cyano group, a carbamoyl group having 1 to 10 carbon atoms in total, an aliphatic oxycarbonyl group having 1 to 8 carbon atoms in total, an aliphatic sulfonyl group having 1 to 8 carbon atoms in total, or a carboxy group; and X2 represents a nitrogen atom or C-CN.
-
Synthese und Antitumorwirkung von Iso-Piritrexim, einem lipophilen Folatantagonisten作者:Reinhard Troschütz、Mario Zink、Thomas DennstedtDOI:10.1002/ardp.19953280612日期:——Synthesewege von Iso‐Piritrexim (12) beschrieben. A) Die Aminomethylierung des Ketons 2 ergibt die Mannich‐Basen 4‐HCI und 5‐HCI, von denen 4, nach sc‐Abtrennung, mit in situ erzeugtem 3,3‐Diaminoacrylnitril (9) zum 2‐Aminonicotinsäurenitril 11 cyslisiert. Der Ringschluß von 11 mit Guanidin führt zum Iso‐PTX(12). B) Durch Reduktion und Oxidation des β‐Ketoesters 15 gelangt man zu einem β‐Keto‐aldehyd
-
A highly effective green catalyst Ni/Cu bimetallic nanoparticles supported by dendritic ligand for chemoselective oxidation and reduction reaction作者:Md. Sayedul Islam、Md. Wahab KhanDOI:10.1007/s11696-020-01480-z日期:2021.6Microscopy (TEM) analysis. These NPs were studied as a heterogeneous catalyst for the chemoselective oxidation of alcohol to the corresponding aldehyde at 30 min and chemoselective reduction of aromatic nitro substituents to the corresponding amino substituents at 20 min, while the Ni/Cu (3:1) NPs were found to be the most effective among other Ni/Cu (1:1) and Ni/Cu (1:3) NPs at room temperature under mildNi和Cu(1:1、1:3、3:1)不同摩尔比的高活性Ni / Cu双金属纳米颗粒(NPs)以及树状配体2,4,6-Tris(di-4-chlorobenzamido )-1,3-二嗪合成成功通过扫描电子显微镜(SEM),电子衍射X射线(EDX),X射线荧光光谱(XRF),X射线衍射(XRD)和透射电子显微镜( TEM)分析。研究了这些NP作为非均相催化剂,用于在30分钟时将醇化学选择性氧化为相应的醛,并在20分钟时将芳族硝基取代基化学选择性还原为相应的氨基取代基,同时发现了Ni / Cu(3:1)NP。在室温和温和条件下,在其他Ni / Cu(1:1)和Ni / Cu(1:3)NP中最有效。镍/铜(3: 图形摘要
-
Mannich-Type C-Nucleosidations with 7-Carba-purines and 4-Aminopyrimidines作者:Ramanarayanan Krishnamurthy、Albert Eschenmoser、Bo Han、Vivek Rajwanshi、Jyoti NandyDOI:10.1055/s-2005-864789日期:——Regioselective Mannich-type C-nucleosidations of pyrroline derivatives 3 and 8 with 7-carba-purines and 4-aminopyrimidines occur under mild conditions to afford C(8)- and C(5)-azanucleosides, respectively.
-
Pyridopyrimidine protein tyrosine phosphatase inhibitors申请人:Berthel Joseph Steven公开号:US20070021445A1公开(公告)日:2007-01-25The present invention comprises pyridopyrimidinediamine compounds of the general formula I: The compounds of the present invention are potent inhibitors of PTP1B. Accordingly, the invention also encompasses pharmaceutical compositions and methods of treating or preventing PTP-1B mediated diseases, including diabetes, obesity, and diabetes-related diseases.
表征谱图
-
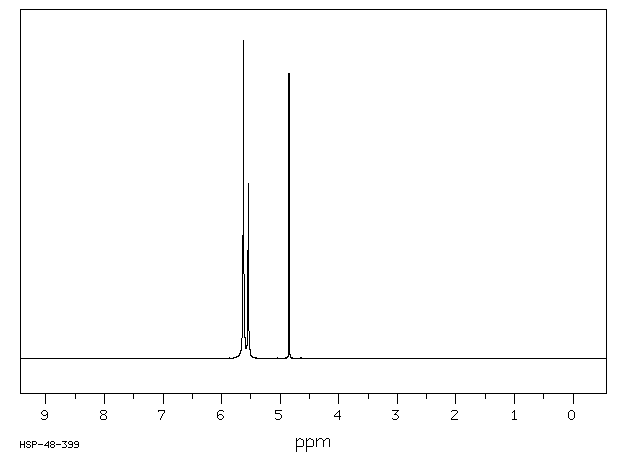
氢谱1HNMR
-
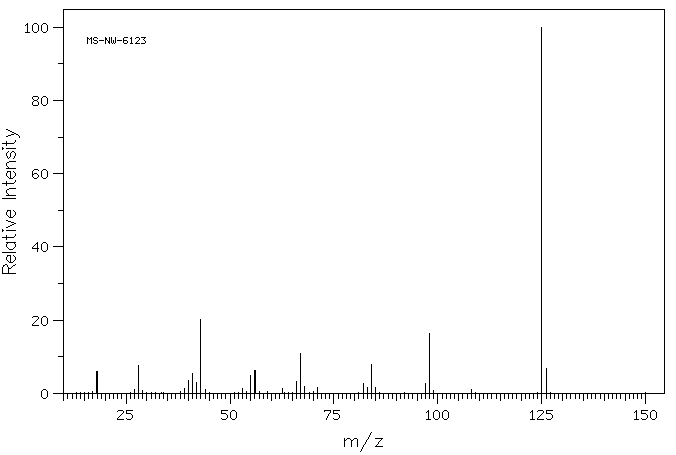
质谱MS
-
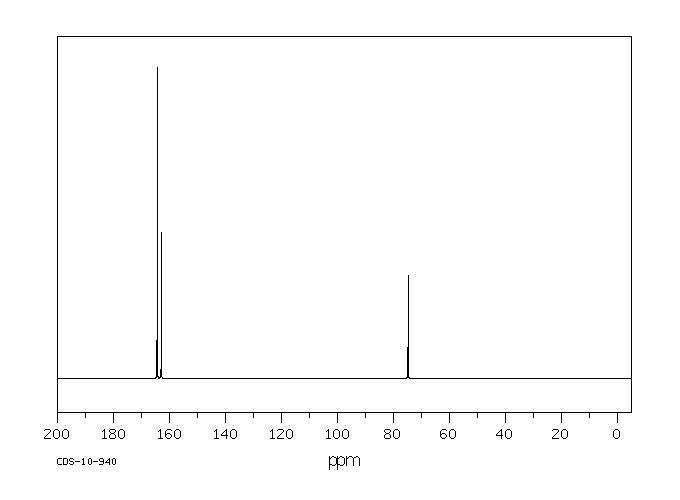
碳谱13CNMR
-
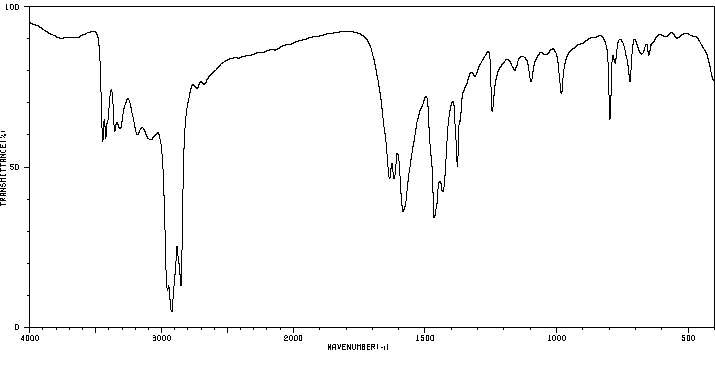
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3