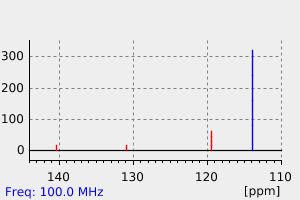
代谢
2,6-DICHLORO-4-NITROANILINE DCNA WAS ABSORBED BY ROOTS OF LETTUCE & TOMATOES GROWN IN TREATED SOIL & WAS TRANSLOCATED TO LEAVES, WHERE IT UNDERWENT RAPID DEGRADATION TO POLAR METABOLITES. TRANSIENT METABOLITES 2,6-DICHLORO-P-PHENYLENEDIAMINE WAS NOT DETECTED.
来源:Hazardous Substances Data Bank (HSDB)