2,6-二硝基苯酚 | 573-56-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
物理描述:2,6-dinitrophenol is a yellow crystalline solid with a sweet musty odor. Sinks and mixes slowly with water. (USCG, 1999)
-
颜色/状态:Pale yellow rhomboid needles or leaflets from dilute alcohol
-
熔点:63.5 °C
-
溶解度:In water, 5164 mg/L at 50 °C
-
密度:1.68 (est) (USCG, 1999)
-
蒸汽密度:6.35 (Air= 1)
-
蒸汽压力:1.2X10-5 mm Hg at 25 °C (est)
-
稳定性/保质期:
-
气味阈值:Detection: 10 mg/L
-
解离常数:pKa = 3.69 at 25 °C
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:13
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:112
-
氢给体数:1
-
氢受体数:5
ADMET
安全信息
-
危险品标志:T,N
-
安全说明:S28,S37,S45,S61
-
危险类别码:R23/24/25,R51/53,R33
-
WGK Germany:3
-
RTECS号:SL2975000
-
海关编码:29089990
-
包装等级:I
-
危险类别:4.1
-
危险品运输编号:UN 1320 4.1/PG 1
SDS
模块 1. 化学品
1.1 产品标识符
: 2,6-二硝基苯酚
产品名称
1.2 鉴别的其他方法
β-Dinitrophenol
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
易燃固体 (类别 1)
模块 16. 其他信息
进一步信息
上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正
确的安全提示来说适用于本品。该信息不代表对此产品性质的保证。
参见发票或包装条的反面。
急性毒性, 经口 (类别 3)
急性毒性, 吸入 (类别 2)
急性毒性, 经皮 (类别 3)
特异性靶器官系统毒性(反复接触), 吸入 (类别 2)
急性水生毒性 (类别 1)
慢性水生毒性 (类别 2)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H228 易燃固体
H301 吞咽会中毒
H311 皮肤接触会中毒
H330 吸入致命。
H373 长期或重复接触可能通过吸入对器官造成伤害。
H400 对水生生物毒性极大。
H411 对水生生物有毒并有长期持续的影响。
警告申明
预防措施
P210 远离热源、火花、明火和热表面。- 禁止吸烟。
P240 容器和接收设备接地。
P241 使用防爆的电气/ 通风/ 照明 设备。
P260 不要吸入粉尘/ 烟/ 气体/ 烟雾/ 蒸汽/ 喷雾。
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P273 避免释放到环境中。
P280 戴防护手套/穿防护服/戴护目镜/戴面罩.
P284 戴呼吸防护装置。
事故响应
P301 + P310 如果吞下去了: 立即呼救解毒中心或医生。
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P310 立即呼叫中毒控制中心或医生.
P320 具体紧急处置(见本标签上提供的急救指导)。
P330 漱口。
P361 立即除去/脱掉所有沾污的衣物。
P363 沾污的衣服清洗后方可再用。
P370 + P378 火灾时: 用干的砂子,干的化学品或耐醇性的泡沫来灭火。
P391 收集溢出物。
安全储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物
干燥时易爆。
干燥时易爆。, 通过皮肤迅速吸收。
模块 3. 成分/组成信息
3.1 物 质
: β-Dinitrophenol
别名
: C6H4N2O5
分子式
: 184.11 g/mol
分子量
组分 浓度或浓度范围
2,6-Dinitrophenol
<=100%
化学文摘登记号(CAS 573-56-8
No.) 209-357-9
EC-编号 609-054-00-5
索引编号
2,4-Dinitrophenol
5-7%
化学文摘登记号(CAS 51-28-5
No.) 200-087-7
EC-编号 609-041-00-4
索引编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 立即将患者送往医院。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
禁止催吐。 切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
中断氧化磷酸化导致代谢升高、氧消耗和产热增加。, …的突然发作, 口渴, 出汗, 恶心, 呕吐, 虚弱, 头晕, 眩晕,
头痛, 食欲减退, 可能发生对肝的伤害。, 皮炎, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
用水喷雾冷却未打开的容器。
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
戴呼吸罩。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 移去所有火源。
人员疏散到安全区域。 避免吸入粉尘。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
一定要避免排放到周围环境中。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
扫掉和铲掉。
围堵溢出,用防电真空清洁器或湿刷子将溢出物收集起来,并放置到容器中去,根据当地规定处理(见第13部
分)。 放入合适的封闭的容器中待处理。
围堵溢出,用防电的真空清洁器或者湿刷子收起,然后装入容器,按照当地法规处理(见第13部分)。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。切勿靠近火源。-严禁烟火。采取措施防止静电积聚。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
对光线敏感 热敏感。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
避免与皮肤、眼睛和衣服接触。 休息前和操作本品后立即洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 阻燃防静电防护服,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N100型(US
)或P3型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 61 - 63 °C
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
根据类别1,此物质或混合物是可燃性固体.
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
暴露在光照下。 加热。
热,火焰和火花。 极端温度和直接日晒。
10.5 不相容的物质
强氧化剂, 强碱, 酰基氯, 酸酐
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
半数致死剂量 (LD50) 腹膜内的 - 小鼠 - 45 mg/kg
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
吸入 - 长期或重复接触可能会对器官造成伤害。
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能致死。 可能引起呼吸道刺激。
摄入 误吞会中毒。
皮肤 如果被皮肤吸收会有毒性 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
中断氧化磷酸化导致代谢升高、氧消耗和产热增加。, …的突然发作, 口渴, 出汗, 恶心, 呕吐, 虚弱, 头晕, 眩晕,
头痛, 食欲减退, 可能发生对肝的伤害。, 皮炎, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: SL2975000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
对水生生物毒性极大。
模块 13. 废弃处置
13.1 废物处理方法
产品
在装备有加力燃烧室和洗刷设备的化学焚烧炉内燃烧处理,特别在点燃的时候要注意,因为此物质是高度易燃
性物质 将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 1320 国际海运危规: 1320 国际空运危规: 1320
14.2 联合国运输名称
欧洲陆运危规: DINITROPHENOL, WETTED
国际海运危规: DINITROPHENOL, WETTED
国际空运危规: Dinitrophenol, wETTed
14.3 运输危险类别
欧洲陆运危规: 4.1 (6.1) 国际海运危规: 4.1 (6.1) 国际空运危规: 4.1 (6.1)
14.4 包裹组
欧洲陆运危规: I 国际海运危规: I 国际空运危规: I
14.5 环境危险
欧洲陆运危规: 是 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 是
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氨基-2,6-二硝基-苯酚 isopicramic acid 17973-92-1 C6H5N3O5 199.123 2,6-二硝基苯甲醚 2,6-dinitroanisole 3535-67-9 C7H6N2O5 198.135 硝苯酚 2-hydroxynitrobenzene 88-75-5 C6H5NO3 139.111 N-(4-羟基-3,5-二硝基苯基)乙酰胺 2,6-dinitro-4-acetylaminophenol 118828-85-6 C8H7N3O6 241.16 —— 2,6-dinitrophenyl 2,4,6-trinitrophenyl ether 103612-93-7 C12H5N5O11 395.199 1,3-二硝基苯 1,3-Dinitrobenzene 99-65-0 C6H4N2O4 168.109 2,3-二硝基苯酚 2,3-dinitrophenol 66-56-8 C6H4N2O5 184.108 4-羟基-3,5-(二硝基苯)胂酸 (4-hydroxy-3,5-dinitro-phenyl)-arsonic acid 6269-50-7 C6H5AsN2O8 308.036 4-羟基-3,5-二硝基苯磺酸 4-hydroxy-3,5-dinitro-benzenesulfonic acid 67329-16-2 C6H4N2O8S 264.172 2,6-二硝基苯胺 2,6-dinitroaniline 606-22-4 C6H5N3O4 183.123 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2 - 氨基- 6 -硝基苯酚 2-amino-6-nitrophenol 603-87-2 C6H6N2O3 154.125 苦味酸 2,4,6-Trinitrophenol 88-89-1 C6H3N3O7 229.106 —— 2,6-dinitrohydroquinone 13985-45-0 C6H4N2O6 200.108 2,6-二硝基苯甲醚 2,6-dinitroanisole 3535-67-9 C7H6N2O5 198.135 4-氯-2,6-二硝基苯酚 4-chloro-2,6-dinitrophenol 88-87-9 C6H3ClN2O5 218.553 —— 2-Methylamino-6-nitro-phenol —— C7H8N2O3 168.152 —— 4-iodo-2,6-dinitrophenol 89488-54-0 C6H3IN2O5 310.005 4-溴-2,6-二硝基苯酚 4-bromo-2,6-dinitrophenol 40466-95-3 C6H3BrN2O5 263.004 2-氨基-4-硝基苯酚 2-hydroxy-5-nitroaniline 99-57-0 C6H6N2O3 154.125 —— 2,6-dinitrophenyl 2,4,6-trinitrophenyl ether 103612-93-7 C12H5N5O11 395.199 —— 2,6-dinitrophenyl acetate 1523-09-7 C8H6N2O6 226.145 —— 2-bromo-4,6-dinitrophenol 2316-50-9 C6H3BrN2O5 263.004 —— N-(2-Hydroxy-3-nitrophenyl)-N'-phenylurea —— C13H11N3O4 273.248 —— 2-chloro-N-(2-hydroxy-3-nitrophenyl)acetamide 85126-68-7 C8H7ClN2O4 230.608 2,6-二硝基苯胺 2,6-dinitroaniline 606-22-4 C6H5N3O4 183.123 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Huebner; Schneider, Zeitschrift fur Chemie, 1871, p. 528摘要:DOI:
-
作为产物:参考文献:名称:Microwave assisted synthesis of nitro phenols from the reaction of phenols with urea nitrate under acid-free conditions摘要:Urea nitrate was found to be an inexpensive, acid-free, and safe nitrating agent that provides mononitration of phenols and substituted phenols in excellent yields with exclusive ortho-selectivity under microwave irradiation. Microwave assisted reactions reduced the reaction times substantially and enhanced the product yields from good to excellent within shorter reaction times. (C) 2014 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2013.12.114
文献信息
-
Alpha2C adrenoreceptor agonists申请人:McCormick D. Kevin公开号:US20070093477A1公开(公告)日:2007-04-26In its many embodiments, the present invention relates to a novel class of phenylmorpholine and phenylthiomorpholine compounds useful as α2C adrenergic receptor agonists, pharmaceutical compositions containing the compounds, and methods of treatment, prevention, inhibition, or amelioration of one or more diseases associated with the α2C adrenergic receptor agonists using such compounds or pharmaceutical compositions.
-
The Anomalous Reactions of Some Fluoronitrobenzenes With Some N,N-Dialkylamines作者:DJ Gale、J Rosevear、JFK WilshireDOI:10.1071/ch9950997日期:——
The reactions of 1-fluoro-2,4- and -2,6-dinitrobenzene with certain N,N- dialkylamines in dimethyl sulfoxide solution in the presence of potassium carbonate give the corresponding dinitrophenyl N,N- dialkylcarbamates as well as the corresponding N,N- dialkyldinitroanilines. The extent of carbamate formation is governed by steric factors. The corresponding reactions of 1-fluoro-4-nitrobenzene with diisopropylamine and di-s-butylamine give poor yields of the corresponding 4-nitrophenyl N,N- dialkylcarbamates but none of the corresponding N,N-dialkyl-4-nitroanilines; in these reactions, the major product is 4,4'-dinitrodiphenyl ether. The 1H and 13C n.m.r. spectra of the 2,4- and 2,6-dinitrophenyl N,N- dialkylcarbamates reveal that their aliphatic protons and carbon atoms are magnetically non-equivalent.
-
Selective Nitration of Aromatic Compounds with Bismuth Subnitrate and Thionyl Chloride作者:Hussni MuathenDOI:10.3390/80700593日期:——Bismuth subnitrate/thionyl chloride have been found to be an efficient combination of reagents for nitration of a wide range of aromatic compounds in dichloromethane. Phenols, in particular, were easily mononitrated and dinitrated with the reagents by controlling the stoichiometry,
-
Magnetically Recyclable Pd/Fe<sub>3</sub>O<sub>4</sub>/g-C<sub>3</sub>N<sub>4</sub> as Efficient Catalyst for the Reduction of Nitrophenol and Suzuki-Miyaura Reaction at Room Temperature作者:Yingwei Yang、Ruiren TangDOI:10.1246/cl.180007日期:2018.4.5the planar structure of g-C3N4. The activity parameter of the catalyst turned out to be excellent (12.4 s−1·mM−1) by fitting the curves derived from the reduction of nitrophenol. Meanwhile, the Suzuki-Miyaura coupling reaction at room temperature processed smoothly with the Pd/Fe3O4/g-C3N4 catalyst. It is meaningful to stress that 2-bromobenzonitrile and p-tolylboronic acid could afford the medical在此,我们提出了一种新型磁性稳定且可回收的催化剂 Pd/Fe3O4/g-C3N4 的设计和合成,该催化剂具有基于片状石墨碳氮化物 (g- ) 的可接近的活性钯纳米粒子 (Pd NPs)。由于具有丰富氮原子的 g- 层状结构,Pd NPs、g- 和磁性粒子 ( ) 通过非共价相互作用紧密连接,促进了 Pd/ /g- 的稳定性并激活了 Pd同时 NP。Pd/ /g- 催化剂具有窄的 Pd NPs 粒径分布 (2.55 nm),显示出 g- 平面结构的维持。通过拟合硝基苯酚还原得到的曲线,该催化剂的活性参数非常好(12.4 s-1·mM-1)。同时,Suzuki-Miyaura 偶联反应在室温下用 Pd/ /g- 催化剂顺利进行。强调2-溴苯甲腈和对甲苯基硼酸可负担医疗...
-
Synthesis and biological evaluation of piperazinyl heterocyclic antagonists of the gonadotropin releasing hormone (GnRH) receptor作者:Matthew D. Vera、Joseph T. Lundquist、Murty V. Chengalvala、Joshua E. Cottom、Irene B. Feingold、Lloyd M. Garrick、Daniel M. Green、Diane B. Hauze、Charles W. Mann、John F. Mehlmann、John F. Rogers、Linda Shanno、Jay E. Wrobel、Jeffrey C. PelletierDOI:10.1016/j.bmcl.2010.02.099日期:2010.4orally active GnRH antagonists based on a 4-piperazinylbenzimidazole template, we sought to investigate the properties of heterocyclic isosteres of the benzimidazole template. We report here the synthesis and biological activity of eight novel scaffolds, including imidazopyridines, benzothiazoles and benzoxazoles. The 2-(4-tert-butylphenyl)-8-(piperazin-1-yl)imidazo[1,2-a]pyridine ring system was shown
表征谱图
-
氢谱1HNMR
-
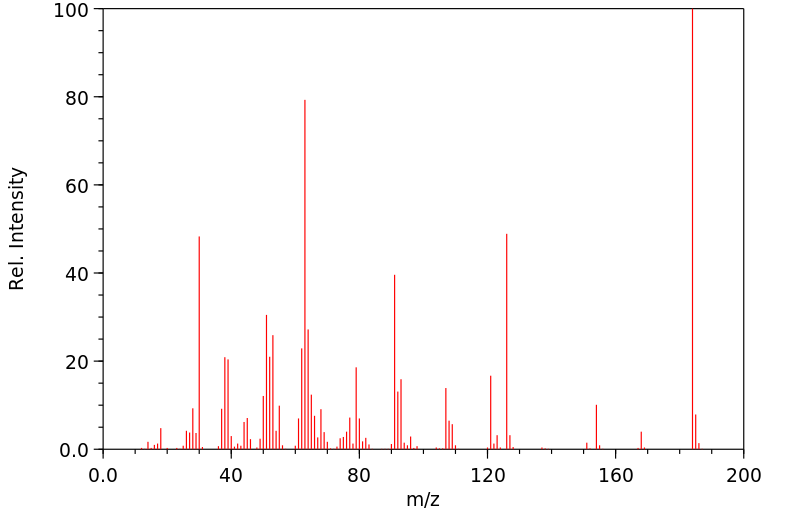
质谱MS
-
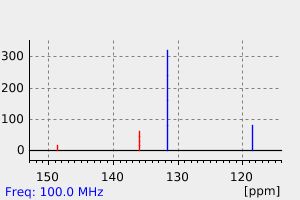
碳谱13CNMR
-
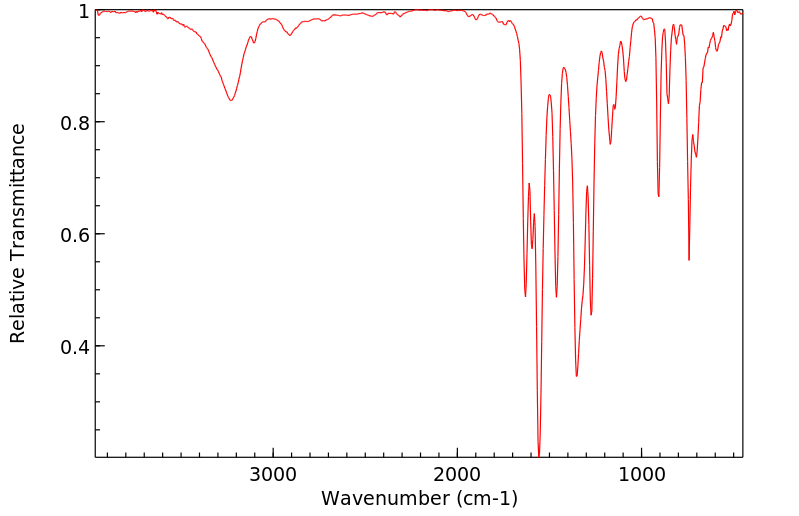
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息