2,6-氯甲酰吡啶 | 3739-94-4
中文名称
2,6-氯甲酰吡啶
中文别名
2,6-吡啶二羰酰氯;2,6-吡啶二甲酰氯
英文名称
2,6-Pyridinedicarbonyl dichloride
英文别名
pyridine-2,6-dicarbonyl dichloride;2,6-pyridinedicarbonyl chloride;2,6-pyridinedicarboxylic acid chloride;2,6-pyridinedicarboxyl chloride;pyridine-2,6-dicarbonyl chloride
CAS
3739-94-4
化学式
C7H3Cl2NO2
mdl
MFCD00006289
分子量
204.012
InChiKey
GWHOGODUVLQCEB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:56-58 °C (lit.)
-
沸点:284 °C (lit.)
-
密度:1.4521 (rough estimate)
-
闪点:284°C
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:47
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S24/25,S26,S36/37/39,S45
-
危险类别码:R34
-
WGK Germany:3
-
海关编码:2933399090
-
危险品运输编号:UN 3261 8/PG 2
-
包装等级:II
-
危险类别:8
-
危险性防范说明:P280,P305+P351+P338,P310
-
危险性描述:H314
-
储存条件:请将产品存放在避光、通风且干燥的地方,并密封保存。
SDS
| Name: | 2 6-Pyridinedicarboxylic Acid Chloride 97% Material Safety Data Sheet |
| Synonym: | 2,6-Pyridinedicarbonyl Dichloride |
| CAS: | 3739-94-4 |
Synonym:2,6-Pyridinedicarbonyl Dichloride
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3739-94-4 | 2,6-Pyridinedicarboxylic Acid Chloride | 97% | 223-125-4 |
Risk Phrases: 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns.Moisture sensitive.Corrosive.Lachrymator (substance which increases the flow of tears).
Potential Health Effects
Eye:
Causes eye burns. Produces irritation, characterized by a burning sensation, redness, tearing, inflammation, and possible corneal injury. Lachrymator (substance which increases the flow of tears).
May cause chemical conjunctivitis and corneal damage.
Skin:
Causes skin burns. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color.
Ingestion:
Causes gastrointestinal tract burns. May cause perforation of the digestive tract. May cause cardiac disturbances. May cause central nervous system effects. May cause systemic effects.
Inhalation:
Causes chemical burns to the respiratory tract. Aspiration may lead to pulmonary edema. May cause cardiac abnormalities. May cause systemic effects. Central nervous system effects, which appear to predominate in acute cases are characterized by abnormal fatigue, memory difficulties and dizziness.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes.
Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray to cool fire-exposed containers. Do NOT get water inside containers. For small fires, use dry chemical, carbon dioxide, or water spray. For large fires, use dry chemical, carbon dioxide, alcohol-resistant foam, or water spray.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation. Do not get water inside containers.
Section 7 - HANDLING and STORAGE
Handling:
Use only in a well-ventilated area. Minimize dust generation and accumulation. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Keep container tightly closed. Do not ingest or inhale. Do not allow contact with water. Discard contaminated shoes. Keep from contact with moist air and steam.
Storage:
Keep container closed when not in use. Corrosives area. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 3739-94-4: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: brown
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 284 deg C @ 760.00mmHg
Freezing/Melting Point: 56.00 - 58.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H3Cl2NO2
Molecular Weight: 204.01
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, moisture, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Moisture, oxidizing agents, strong bases.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3739-94-4 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,6-Pyridinedicarboxylic Acid Chloride - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE SOLID, N.O.S.*
Hazard Class: 8
UN Number: 1759
Packing Group: II
IMO
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing Group: II
RID/ADR
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 34 Causes burns.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 3739-94-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 3739-94-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3739-94-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 甲基6-(氯甲酰基)-2-吡啶羧酸酯 methyl 6-(chlorocarbonyl)picolinate 94111-79-2 C8H6ClNO3 199.594 吡啶-2,6-二甲醛 2,5-diformylpyridine 5431-44-7 C7H5NO2 135.122 —— ethyl 6-(chlorocarbonyl)-pyridine-2-carboxylate 851585-91-6 C9H8ClNO3 213.62
反应信息
-
作为反应物:描述:参考文献:名称:吡啶2,6-二硫代羧酸及其金属配合物:新德里Metalloβ-内酰胺酶-1的新抑制剂。摘要:关键字: NDM-1抑制剂;海洋来源的链霉菌;耐碳青霉烯的肠杆菌科; 金属螯合剂DOI:10.3390/md18060295
-
作为产物:描述:参考文献:名称:螯合双(亚氨基)吡啶钴配合物:Li+阳离子活化生成乙烯聚合催化剂的合成、还原和证据摘要:用FeCl 2 或CoCl 2 处理双(亚氨基苄基)吡啶螯合席夫碱配体8 (ligPh) 产生相应的(ligPh) MCl 2 配合物9 (Fe)和10 (Co)。10与甲基锂或“丁二烯-镁”的反应导致还原得到相应的(ligPh)Co(I)Cl产物11。类似地,双(芳基亚氨基乙基)吡啶配体(ligMe)与CoCl2反应得到(ligMe) )CoCl2 (12)。通过用“丁二烯-镁”处理来还原成(ligMe)CoCl (13)。配合物 13 在甲苯中与 Li[B(C6F5)4] 反应,然后用吡啶处理,得到 [(ligMe)Co+-吡啶] (15)。Co(II) 络合物 10 或 12 与约 3 摩尔当量的甲基锂分别得到钴 (I) 配合物 16 和 17。用Li[B(C6F5)4]处理(ligMe)CoCH3(17)得到低活性的乙烯聚合催化剂。同样,在用化学计量量的 Li[B(C6F5)4 处理后,复合物DOI:10.1021/ja052129k
文献信息
-
Iron-Doped Single-Walled Carbon Nanotubes as New Heterogeneous and Highly Efficient Catalyst for Acylation of Alcohols, Phenols, Carboxylic Acids and Amines under Solvent-Free Conditions作者:Hashem Sharghi、Mahboubeh Jokar、Mohammad Mahdi DoroodmandDOI:10.1002/adsc.201000365日期:2011.2.11single-walled carbon nanotubes (Fe/SWCNTs) represent an efficient and new heterogeneous reusable catalyst for the acylation of a variety of alcohols, phenols, carboxylic acids and amines with acid chlorides or acid anhydrides under solvent-free conditions. The reactions of various primary, secondary, tertiary, and benzylic alcohols, diols, phenols, as well as aromatic and aliphatic amines give acylated
-
METHOD FOR THE CATALYTIC REDUCTION OF ACID CHLORIDES AND IMIDOYL CHLORIDES申请人:Nikonov Georgii公开号:US20140228579A1公开(公告)日:2014-08-14The present application relates to methods for the catalytic reduction of acid chlorides and/or imidoyl chlorides. The methods comprise reacting the acid chloride or imidoyl chloride with a silane reducing agent in the presence of a catalyst such as [Cp(Pr i 3 P)Ru(NCMe) 2 ] + [PF 6 ] − .
-
Rhodium(III)-Catalyzed Oxidative Olefination of Picolinamides: Convenient Synthesis of 3-Alkenylpicolinamides作者:Jun Zhou、Bo Li、Zhen-Chao Qian、Bing-Feng ShiDOI:10.1002/adsc.201300895日期:2014.3.24A rhodium(III)‐catalyzed selective olefination of picolinamide derivatives has been developed. The reaction shows high regioselectivity, low catalyst loading (0.5 mol%), high yield and good functional group tolerance, providing a convenient strategy for the synthesis of 3‐alkenylpicolinamides.
-
Synthesis of Fluorogenic Arylureas and Amides and Their Interaction with Amines: A Competition between Turn-on Fluorescence and Organic Radicals on the Way to a Smart Label for Fish Freshness作者:Javier García-Tojal、José V. Cuevas、María-Josefa Rojo、Borja Díaz de Greñu、Carla Hernando-Muñoz、José García-Calvo、Mateo M. Salgado、Tomás TorrobaDOI:10.3390/molecules26051404日期:——and their interaction with primary or secondary amines under air and light in organic-aqueous mixtures to give rise to a new class of persistent organic radicals, described on the basis of their electron paramagnetic resonance (EPR), as well as UV–vis, fluorescence, NMR, and quantum mechanics calculations, and their prospective use as multi-signal reporters in a smart label for fish freshness.
-
Rational Tuning of Melting Entropies for Designing Luminescent Lanthanide-Containing Thermotropic Liquid Crystals at Room Temperature作者:Aude Escande、Laure Guénée、Homayoun Nozary、Gérald Bernardinelli、Frédéric Gumy、Annina Aebischer、Jean-Claude G. Bünzli、Bertrand Donnio、Daniel Guillon、Claude PiguetDOI:10.1002/chem.200700560日期:2007.10.26room-temperature mesomorphism is detected. An enthalpically unbalanced large melting entropy (DeltaSmL11=226 J mol(-1) K(-1)) results from the programmed microsegregation induced in the crystalline phase, a phenomenon which is maintained in the associated lanthanide complexes [Ln(L11)(NO3)3] and [Ln(L11)(CF3CO2)3]2. Low-temperature melting processes (-43
表征谱图
-
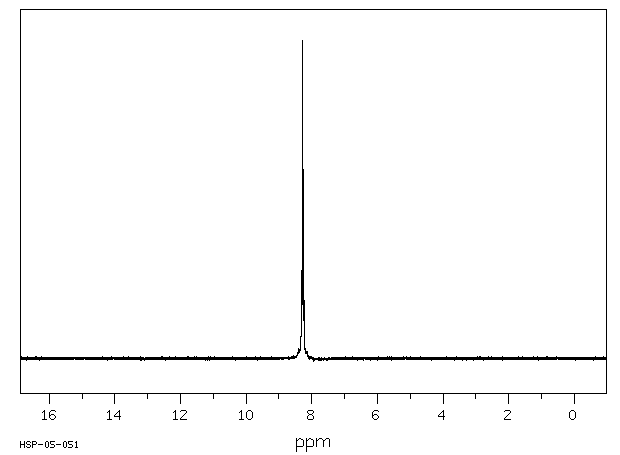
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
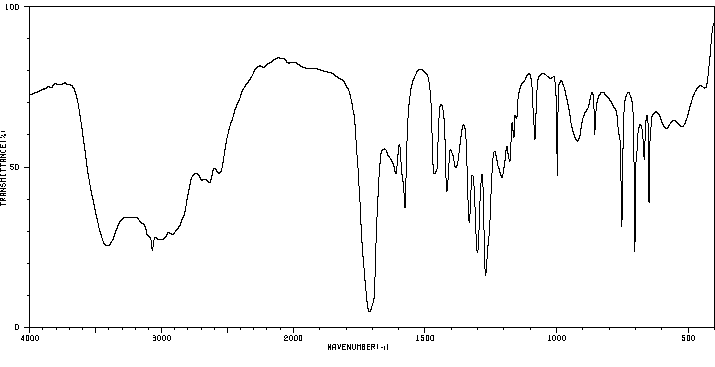
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-