2,7-二甲基-1,8-萘啶 | 14903-78-7
中文名称
2,7-二甲基-1,8-萘啶
中文别名
——
英文名称
2,7-dimethyl-[1,8]naphthyridine
英文别名
2,7-Dimethyl-1,8-naphthyridine
CAS
14903-78-7
化学式
C10H10N2
mdl
MFCD08753877
分子量
158.203
InChiKey
IVOPVBBXMWVCHV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:194-195 °C
-
沸点:259.7±35.0 °C(Predicted)
-
密度:1.109±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:25.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2933990090
-
危险性防范说明:P261,P264,P271,P280,P302+P352,P304+P340,P305+P351+P338,P312,P362,P403+P233,P501
-
危险性描述:H315,H319,H335
-
储存条件:室温且干燥
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-[1,8]-萘啶 2-methyl-[1,8]naphthyridine 1569-16-0 C9H8N2 144.176 —— 2,7-dimethyl-1,8-naphthyridine-4-ol 53051-92-6 C10H10N2O 174.202 4-氯-2,7-二甲基-1,8-二氮杂萘 4-chloro-2,7-dimethyl-1,8-naphthyridine 84670-41-7 C10H9ClN2 192.648 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,8-naphthyridine-2,7-dicarboxaldehyde 65896-28-8 C10H6N2O2 186.17 —— 2-(bromomethyl)-7-methyl-1,8-naphthyridine —— C10H9BrN2 237.099 —— 2,7-Bis(hydroxymethyl)-1,8-naphthyridine 125902-22-9 C10H10N2O2 190.202 —— 2,7-Bis(trichloromethyl)-1,8-naphthyridine 125902-20-7 C10H4Cl6N2 364.873 —— 1,8-naphthyridine-2,7-dicarboxylic acid 84670-42-8 C10H6N2O4 218.169 —— 1,8-naphthyridine-2,7-dicarbonyl dichloride 84670-43-9 C10H4Cl2N2O2 255.06 —— 2,7-bis(N,N-diethyl-aminomethyl)-1,8-naphthyridine 313672-73-0 C18H28N4 300.447 —— 2,7-bis[bis(2-pyridylmethyl)aminomethyl]-1,8-naphthyridine 301153-11-7 C34H32N8 552.682 —— 2,7-Bis(methoxycarbonyl)-1,8-naphthyridine 125902-21-8 C12H10N2O4 246.222 —— 3,6,9,12,15-pentaoxa-23,25-diazatricyclo<15.5.3.020.24>pentacosa-17,19,21,1(23),24-pentaene-2,16-dione 84670-45-1 C18H20N2O7 376.366 —— 3,6,9,12-tetraoxa-20,22-diazatricyclo<12.5.3.017.21>docosa-14,16,18,1(20),21-pentaene-2,13-dione 84670-44-0 C16H16N2O6 332.313 - 1
- 2
反应信息
-
作为反应物:描述:2,7-二甲基-1,8-萘啶 在 4-二甲氨基吡啶 、 selenium(IV) oxide 、 1-羟基苯并三唑 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 作用下, 以 1,4-二氧六环 、 N,N-二甲基甲酰胺 为溶剂, 反应 39.0h, 生成 N,N'-di(quinolin-3-yl)-1,8-naphthyridine-2,7-dicarboxamide参考文献:名称:Selective G-quadruplex DNA Stabilizing Agents Based on Bisquinolinium and Bispyridinium Derivatives of 1,8-Naphthyridine摘要:Various biologically relevant G-quadruplex DNA structures offer a platform for therapeutic intervention for altering the gene expression or by halting the function of proteins associated with telomeres. One of the prominent strategies to explore the therapeutic potential of quadruplex DNA structures is by stabilizing them with small molecule ligands. Here we report the synthesis of bisquinolinium and bispyridinium derivatives of 1,8-naphthyridine and their interaction with human telomeric DNA and promoter G-quadruplex forming DNAs. The interactions of ligands with quadruplex forming DNAs were studied by various biophysical, biochemical, and computational methods. Results indicated that bisquinolinium ligands bind tightly and selectively to quadruplex DNAs at low ligand concentration (similar to 0.2-0.4 mu M). Furthermore, thermal melting studies revealed that ligands imparted higher stabilization for quadruplex DNA (an increase in the T-m of up to 21 degrees C for human telomeric G-quadruplex DNA and >25 degrees C for promoter G-quadruplex DNAs) than duplex DNA (Delta T-m < 1.6 degrees C). Molecular dynamics simulations revealed that the end-stacking binding mode was favored for ligands with low binding free energy. Taken together, the results indicate that the naphthyridine-based ligands with quinolinium and pyridinium side chains form a promising class of quadruplex DNA stabilizing agents having high selectivity for quadruplex DNA structures over duplex DNA structures.DOI:10.1021/jo201816g
-
作为产物:描述:2-(特戊酰氨基)吡啶-3-醛 在 盐酸 、 potassium permanganate 、 potassium hydroxide 作用下, 以 甲醇 、 水 、 丙酮 为溶剂, 生成 2,7-二甲基-1,8-萘啶参考文献:名称:具有 1,8-萘啶基双(甲硅烷基)支撑配体的二钌配合物:含有 RuII2(μ-H)2 和 RuI2 核的配合物的合成和结构摘要:我们设计了一种新型的基于萘啶的支持配体,它涉及 2,7 位的两个甲硅烷基配位部分,t-BuNBSi,用于合成双核金属配合物。配体前体 t-BuNBSi(H)2 (1) 与 Ru3(CO)12 反应得到二-μ-氢化二钌 (II,II) 配合物 (t-BuNBSi)Ru2(μ-H)2(CO)4 (2). 光照射 2 导致形成二钌 (I, I) 络合物 (t-BuNBSi)Ru2(CO)6 (3)。2 的 Si-Ru-Ru-Si 连接采用锯齿形排列,而 3 的连接采用大致线性的排列。DOI:10.1246/cl.171099
文献信息
-
Facile Synthesis of 6-Trichloromethylpterin and 2-Chloro-3-trichloromethylquinoxaline along with a Library of Trichloromethyl Heterocycles Using<i>N</i>-Chlorosuccinimide and Triphenyl Phosphine作者:Shyamaprosad Goswami、Annada C. Maity、Hoong-Kun FunDOI:10.1246/cl.2007.552日期:2007.4.5A general synthesis of 6-trichloromethylpterin, 2-chloro-3-trichloromethylquinoxaline and 2-amino-7-trichloromethyl-1,8-naphthyridine along with a series of trichloromethyl heterocycles (1–11) has been reported in one-pot mild neutral condition in good yield. This method is compared with the usual method using phosphorus pentachloride in phosphorus oxychloride.
-
A Proton-Responsive Annulated Mesoionic Carbene (MIC) Scaffold on Ir Complex for Proton/Hydride Shuttle: An Experimental and Computational Investigation on Reductive Amination of Aldehyde作者:Pragati Pandey、Prosenjit Daw、Noor U Din Reshi、Kira R. Ehmann、Markus Hölscher、Walter Leitner、Jitendra K. BeraDOI:10.1021/acs.organomet.0c00568日期:2020.11.9A Cp*Ir(III) complex (1) bearing a proton-responsive hydroxy unit on an annulated imidazo[1,2-a][1,8]naphthyridine based mesoionic carbene scaffold was synthesized by two different synthetic routes. The molecular structure of 1 revealed an anionic lactam form of the ligand. The acid–base equilibrium between the lactam-lactim tautomers on the ligand scaffold was examined by 1H NMR and UV–vis spectra通过两种不同的合成途径合成了在环状咪唑并[1,2- a ] [1,8]萘吡啶基介电卡宾骨架上带有质子响应性羟基单元的Cp * Ir(III)配合物(1)。的分子结构1揭示了配体的阴离子内酰胺形式。通过1 H NMR和UV-vis光谱检查了配体支架上内酰胺-内酯互变异构体之间的酸碱平衡。估算内酰胺形式为1的附件-OH基团的p K a,以评估催化剂的质子转移性能。1的催化功效通过使用三种不同的氢源(分子H 2,i PrOH / KO t Bu组合和HCOOH / Et 3 N(5:2)共沸混合物)评估醛的还原胺化反应。发现在三种不同的氢化方法中,HCOOH / Et 3 N(5:2)共沸混合物是最好的。在反应条件下,催化剂1在羰基上化学选择性地氢化亚胺。使用HCOOH / Et 3 N(5:2)共沸混合物将一系列醛还原为相应的仲胺。此外,催化剂1在还原多种N-杂环亚胺衍生物方面显示出高效率。提
-
<i>N</i>-bromosuccinimide reactions of some heterocycles in the presence or absence of water: An overview of ring versus side chain bromination for the synthesis of important brominated heterocyclic synthons作者:Shyamaprosad Goswami、Kumaresh Ghosh、Reshmi Mukherjee、Avijit Kumar Adak、Ajit Kumar MahapatraDOI:10.1002/jhet.5570380125日期:2001.1Reactions of various heterocycles 1–6 with N-bromosuccinimide in the presence or absence of water have been studied for side chain versus ring bromination to afford some new and important heterocyclic synthons. Interestingly, the N-bromosuccinimide reaction in the presence of perchloric acid, a new condition, affords exclusively the new dibromo aminopicoline 1f, which is not obtained by other presently
-
Side Chain Bromination of Mono and Dimethyl Heteroaromatic and Aromatic Compounds by Solid Phase<i>N</i>-Bromosuccinimide Reaction without Radical Initiator under Microwave作者:Shyamaprosad Goswami、Swapan Dey、Subrata Jana、Avijit Kumar AdakDOI:10.1246/cl.2004.916日期:2004.7A series of side chain mono and dibromo derivatives of mono and dimethyl heteroaromatic and aromatic compounds (1–17) were synthesized by one step solid phase N-bromosuccinimide (NBS) reaction with...
-
Rare earth acetylacetonate complexes with 2,7-dimethyl-1,8-naphthyridine作者:M. NgSee、H.W. Latz、D.G. HendrickerDOI:10.1016/0022-1902(77)80435-1日期:1977.1Rare earth acetoacetonate complexes of the type Ln(acac)32,7-dimethyl-1,8-naphthyridine where Ln = Pr Yb have been synthesized. The compounds have been characterized by elemental analyses, molar conductances and IR spectra (4000-200 cm−1). The molar conductances and IR spectra show all ligands are coordinated to the metals. Evidence suggesting a dodecahedral geometry for these 8-coordinate complexes合成了Ln(acac)3 2,7-二甲基-1,8-萘啶型的稀土乙酰丙酮化物络合物,其中Ln = PrYb。这些化合物已通过元素分析,摩尔电导和IR光谱(4000-200 cm -1)进行了表征。摩尔电导和红外光谱显示所有配体均与金属配位。有证据表明这些八坐标复合物包含四元和五元螯合环的十二面体几何结构。
表征谱图
-
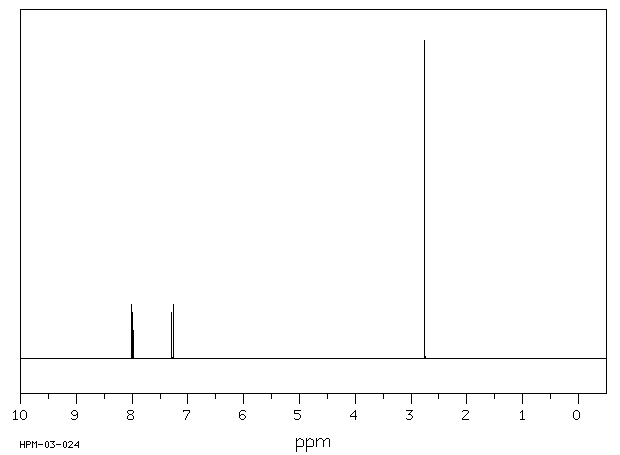
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮