(-)-(R)-N,S-dimethyl-S-phenylsulfoximine | 80482-67-3
中文名称
——
中文别名
——
英文名称
(-)-(R)-N,S-dimethyl-S-phenylsulfoximine
英文别名
R-(-)-N,S-Dimethyl-S-phenylsulfoximine;methyl-methylimino-oxo-phenyl-λ6-sulfane
CAS
80482-67-3
化学式
C8H11NOS
mdl
——
分子量
169.247
InChiKey
OQWUXWSLVBGOIX-LLVKDONJSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:37.8
-
氢给体数:0
-
氢受体数:2
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 S-甲基-S-苯基-N-甲基亚砜亚胺 N,S-dimethyl-S-phenylsulfoximine 30004-67-2 C8H11NOS 169.247 (R)-(-)-S-甲基-S-苯亚砜亚胺 (R)-S-methyl-S-phenylsulfoximine 60933-65-5 C7H9NOS 155.221
反应信息
-
作为反应物:描述:(-)-(R)-N,S-dimethyl-S-phenylsulfoximine 在 咪唑 、 正丁基锂 、 sodium methylate 作用下, 以 四氢呋喃 、 正己烷 、 二氯甲烷 、 N,N-二甲基甲酰胺 为溶剂, 反应 146.5h, 生成参考文献:名称:通过甲硅烷氧基烯基氨基亚砜盐的α-消除和迁移环化不对称合成 2,3-二氢呋喃和不饱和双环四氢呋喃。手性双取代β-甲硅烷氧基亚烷基卡宾的生成和分子内O,Si-键插入摘要:用醛处理衍生自 β-甲基取代的无环烯丙基亚砜亚胺 13a 和 13b 的双(烯丙基亚砜亚胺)钛络合物,以高选择性得到相应的亚砜亚胺取代的高烯丙醇,其被分离为甲硅烷基醚 15a-h。亚砜亚胺 15a-h 的甲基化得到氨基亚砜盐 5a-h,在用 LiN(H)tBu 处理后,以高产率得到对映体和非对映体纯甲硅烷基取代的 2,3-二氢呋喃 4a-h。用 p-MeOC(6)H(4)CHO 处理衍生自环状烯丙基亚砜亚胺 17a、17b 和 ent-17c 的钛配合物,以高选择性提供相应的亚砜亚胺取代环状高烯丙醇,分离为甲硅烷基醚分别参见 18a、18b 和 ent-18c。亚砜亚胺 18a、18b、和 ent-18c 分别提供氨基锍盐 8a、8b 和 ent-8c,其用 LiN(H)t-Bu 处理得到对映和非对映异构纯稠合双环 2,3-二氢呋喃 6a、6b 和 ent- 6c,分别以良好的产量。提出 1-烯基氨基锍盐DOI:10.1021/ja030501v
-
作为产物:参考文献:名称:N,S-dimethyl-s-phenylsulfoximine摘要:DOI:10.1016/s0040-4020(01)82409-1
-
作为试剂:描述:(+/-)-3-Methoxy-13β-ethylgona-1,3,5(10)-trien-17-on 在 正丁基锂 、 (-)-(R)-N,S-dimethyl-S-phenylsulfoximine 作用下, 以 四氢呋喃 为溶剂, 生成 、 (+-)-3-Methoxy-13β-ethylgona-1,3,5(10)-trien-17-on参考文献:名称:Sulfoximine-mediated resolutions of ketones摘要:DOI:10.1021/ja00378a048
文献信息
-
Cross‐Coupling Reaction of Alkenyl Sulfoximines and Alkenyl Aminosulfoxonium Salts with Organozincs by Dual Nickel Catalysis and Lewis Acid Promotion作者:Irene Erdelmeier、Gerd Bülow、Chang‐Wan Woo、Jürgen Decker、Gerhard Raabe、Hans‐Joachim GaisDOI:10.1002/chem.201901163日期:2019.6.21atom. CCR of axially chiral alkenyl sulfoximines with Ni(PPh3)2Cl2 as a precatalyst and ZnPh2 does not require salt promotion and is stereoretentive. The reaction with Zn(CH2SiMe3)2, however, demands salt promotion and is not stereoretentive. CCR of axially chiral α‐methylated alkenyl sulfoximines afforded persubstituted axially chiral alkenes with high selectivity. Alkenyl (N‐triflyl)sulfoximines本文描述了环外,轴向手性和无环烯基(N-甲基)亚磺酰亚胺与烷基锌和芳基锌的交叉偶联反应(CCR)。CCR通常需要双重Ni催化和MgBr 2促进作用,这在乙醚中有效,但在THF中无效。NMR光谱显示,MgBr 2在乙醚中使烯基亚砜亚胺络合,这表明通过核真菌活化可加快氧化加成反应。烯基亚砜肟的CCR通常在Ni(dppp)Cl 2作为前催化剂和MgBr 2的存在下进行在C和S原子上具有高度立体保留的烷基和芳基锌。以Ni(PPh 3)2 Cl 2为前催化剂和ZnPh 2的轴向手性烯基亚磺酰亚胺的CCR不需要盐促进,并且具有立体保持性。但是,与Zn(CH 2 SiMe 3)2的反应需要盐促进,并且不是立体保持性的。轴向手性α-甲基链烯基亚砜亚胺的CCR可提供高选择性的全取代轴向手性烯烃。烯基(N-三氟乙)亚砜肟与格利雅试剂和Ni(PPh 3)2 Cl 2参与立体保持性CCR。镍催化和MgBr 2的促进的CCR
-
Sulfoximine-directed osmylation: synthesis of enantiomerically pure dihydroxycycloalkanones作者:Carl R. Johnson、Michael R. BarbachynDOI:10.1021/ja00320a053日期:1984.4On prepare une serie de dihydroxy-2,3 cyclohexanones et cyclo pentanones di- ou trisubstituees, a partir des cyclenones correspondantes par reaction avec le N-methyl S-methyl S-phenyl sulfoximide关于制备 une serie de dihydroxy-2,3 cyclohexones et cyclopentanones di-ou trisubstituees, a partir des cyclenones相应的反应 avec le N-methyl S-methyl S-phenyl sulfoximide
-
Total synthesis of the cytotoxic macrocycle (+)-hitachimycin作者:Amos B. Smith、Thomas A. Rano、Noritaka Chida、Gary A. Sulikowski、John L. WoodDOI:10.1021/ja00047a008日期:1992.10The first total synthesis of the antitumor antibiotic (+)-hitachimycin (a.k.a. stubomycin) (1) has been achieved in 22 steps and 1.1% overall yield. The cornerstone of the synthetic strategy was a highly stereoselective three-component coupling of (-)-5-methoxycyclopentenone (4) with a zincate derived from vinyl iodide 3a and aldehyde (-)-51
-
Asymmetric Synthesis of the Highly Potent Anti-Metastatic Prostacyclin Analogue Cicaprost and Its Isomer Isocicaprost作者:Marco Lerm、Hans-Joachim Gais、Kejun Cheng、Cornelia VermeerenDOI:10.1021/ja030200l日期:2003.8.1intermediate carrying a carbonyl group at C6. Asymmetric syntheses of the bicyclic C6-C14 ethynyl building blocks were carried out starting from achiral bicyclic C6-C12 ketones by using the chiral lithium amide method. In the course of these syntheses, a new method for the introduction of an ethynyl group at the alpha-position of the carbonyl group of a ketone with formation of the corresponding homopropargylic描述了通过新途径不对称合成抗转移前列环素类似物西卡前列素及其异构体异西卡前列素的正式异构体。这些合成的关键步骤是将手性双环 C6-C14 乙炔基结构单元与手性 C15-C21 ω-侧链酰胺结构单元偶联,形成目标分子的 C14-C15 键。由此获得的在侧链的 C15 处带有羰基的 C6-C21 中间体的高度立体选择性还原是通过手性 oxazaborolidine 方法完成的。手性膦酰基乙酸酯方法用于将 α 侧链高度立体选择性地连接到在 C6 处带有羰基的双环 C6-C21 中间体。通过使用手性氨基化锂方法,从非手性双环 C6-C12 酮开始,进行双环 C6-C14 乙炔基结构单元的不对称合成。在这些合成过程中,设计了一种在酮的羰基的 α 位引入乙炔基并形成相应的高炔丙醇的新方法。其关键步骤是相应的甲硅烷基烯醇醚与氯醛的羟醛反应和消除三氯甲醇衍生物并形成乙炔基。此外,还实现了一种新的醛到末端炔烃的转化。其关键步骤是将醛转化为相应的
-
Diastereoselective additions of lithiated N-tertbutyldiphenylsilyl- S-methyl-S-phenylsulfoximine to imines and aldehydes作者:Stephen G. Pyne、Branko DikicDOI:10.1016/s0040-4039(00)97851-1日期:1990.1Lithiated N-tert-butyldiphenylsilyl-S-methyl-S-phenylsulfoximine undergoes 1,2 addition to imines (R1CHNPh) and aldehydes (RCHO) to give diastereomeric adducts. The diastereoselection of the former reactions is dependent on the steric demand of the imine substituent R1, while that of the latter is independent of the steric demand of the aldehyde substituent R.
表征谱图
-
氢谱1HNMR
-
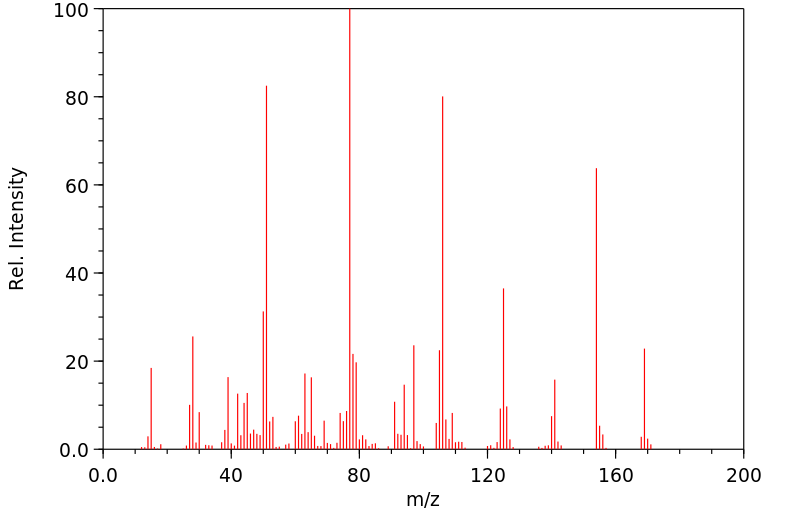
质谱MS
-
碳谱13CNMR
-
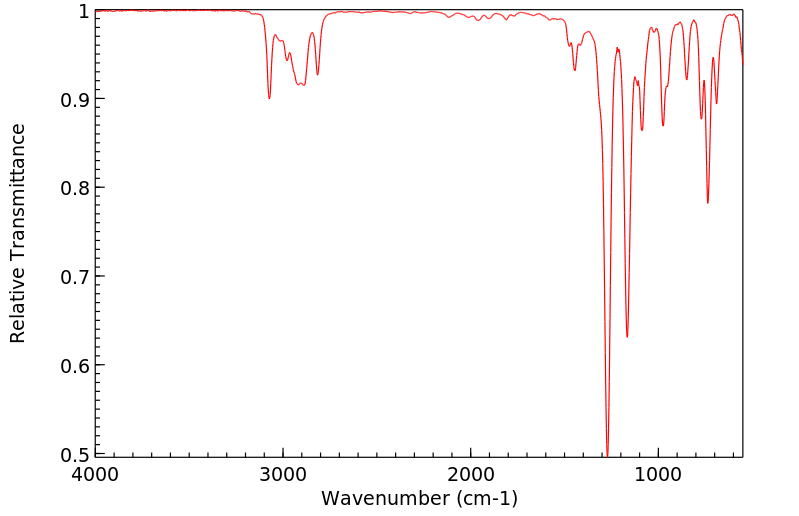
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫