(E)-ethyl 2-benzoyl-3-phenylacrylate | 39626-31-8
分子结构分类
中文名称
——
中文别名
——
英文名称
(E)-ethyl 2-benzoyl-3-phenylacrylate
英文别名
ethyl 2-benzoyl-3-phenylacrylate;ethyl (E)-2-benzoyl-3-phenylacrylate;ethyl (2E)-2-benzoyl-3-phenylacrylate;Benzenepropanoic acid, beta-oxo-alpha-(phenylmethylene)-, ethyl ester;ethyl (E)-2-benzoyl-3-phenylprop-2-enoate
CAS
39626-31-8
化学式
C18H16O3
mdl
——
分子量
280.323
InChiKey
BHKXLHNAIARBLO-DTQAZKPQSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:21
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-ethyl 2-(hydroxy(phenyl)methyl)-3-phenylprop-2-enoate 1033302-66-7 C18H18O3 282.339
反应信息
-
作为反应物:描述:(E)-ethyl 2-benzoyl-3-phenylacrylate 在 sodium tetrahydroborate 、 cerium(III) chloride heptahydrate 作用下, 以 甲醇 为溶剂, 以60%的产率得到(E)-ethyl 2-(hydroxy(phenyl)methyl)-3-phenylprop-2-enoate参考文献:名称:取代基对1,3-二芳基烯丙基阳离子形成高取代茚基的4π-电环选择性的影响摘要:在路易斯酸存在下通过电离相应的烯丙醇而生成的差异取代的1,3-二芳基取代的烯丙基阳离子经历化学选择性和区域选择性电环化反应以生成1-芳基-1 H-茚。仅具有2-取代基且2-酯和2-烷基取代基均被耐受的烯丙基阳离子才发生电环化。通常,吸电子取代基的存在使环失活并不利于环化。相反,含有给电子取代基的体系的环化选择性取决于给电子基团的性质和位置。在间位的供电子取代基特别有利于环化。如亲电子芳族取代反应所建议的,环化选择性与计算的电子密度没有明显的相关性。然而,通过气相(B3LYP / 6-31G * + ZPVE)比较环化相对速率所确定的计算选择性与观察到的选择性非常吻合。环化计算的过渡态结构与阳离子π4一致一个conrotatory electrocyclization机制。在一些涉及更多电子缺陷系统的情况下,最初形成的1 H-茚进行后续的烯烃异构化为3 H-茚。在一个实例中,发生不寻常的二聚DOI:10.1021/jo100275q
-
作为产物:描述:(Z)-N-benzylidenecyclohexanamine oxide 在 碘甲烷 作用下, 以 四氢呋喃 为溶剂, 反应 18.0h, 生成 (E)-ethyl 2-benzoyl-3-phenylacrylate参考文献:名称:Ring-Opening of 4-Isoxazolines: Competitive Formation of Enamino Derivatives and a,b-Enones摘要:Ring-opening of 3-substituted 4-isoxazolines, proceeding through the intermediate isoxazolinium salts, follows two competing reaction pathways leading to alpha,beta-enones and enamines respectively. The rearrangement courses can be controlled as a function of substitution pattern and experimental conditions.DOI:10.3987/com-92-6223
文献信息
-
Indium(III)-Catalyzed Knoevenagel Condensation of Aldehydes and Activated Methylenes Using Acetic Anhydride as a Promoter作者:Yohei Ogiwara、Keita Takahashi、Takefumi Kitazawa、Norio SakaiDOI:10.1021/acs.joc.5b00011日期:2015.3.20amount of InCl3 and acetic anhydride remarkably promotes the Knoevenagel condensation of a variety of aldehydes and activated methylene compounds. This catalytic system accommodates aromatic aldehydes containing a variety of electron-donating and -withdrawing groups, heteroaromatic aldehydes, conjugate aldehydes, and aliphatic aldehydes. Central to successfully driving the condensation series is the formation
-
CBr<sub>4</sub> as a Halogen Bond Donor Catalyst for the Selective Activation of Benzaldehydes to Synthesize α,β-Unsaturated Ketones作者:Imran Kazi、Somraj Guha、Govindasamy SekarDOI:10.1021/acs.orglett.7b00348日期:2017.3.3CBr4 has been employed as a halogen bond donor catalyst for the selective activation of aldehyde, to achieve an efficient solvent- and metal-free C═C bond forming reaction in the presence of strong acid sensitive groups such as methoxy, cyanide, ester, and ketal for the synthesis of α,β-unsaturated ketones. This unique capability of CBr4 to act as a halogen bond donor has been explored and established
-
Asymmetric Total Synthesis of (−)-Plicatic Acid via a Highly Enantioselective and Diastereoselective Nucleophilic Epoxidation of Acyclic Trisubstitued Olefins作者:Bing-Feng Sun、Ran Hong、Yan-Biao Kang、Li DengDOI:10.1021/ja9039407日期:2009.8.5The first total synthesis of (-)-plicatic acid has been achieved by a concise and enantioselective route. In this synthesis, a conceptually new strategy featuring an asymmetric epoxidation-intramolecular epoxy-ring-opening Friedel-Crafts reaction sequence was developed for the stereoselective construction of the 2,7'-cyclolignane skeleton bearing contiguous quaternary-quaternary-tertiary stereocenters(-)-plicatic acid 的首次全合成是通过简洁的对映选择性路线实现的。在该合成中,开发了一种具有不对称环氧化-分子内环氧-开环 Friedel-Crafts 反应序列的概念性新策略,用于立体选择性构建带有连续四元-四元-叔立体中心的 2,7'-环木脂烷骨架。这一策略的实施是通过使用 TADOOH 开发用于 Seebach 环氧化的改进方案来实现的,该方案提供了前所未有的、高度对映选择性和非对映选择性环氧化,其中包括一系列 α-羰基-β-取代的丙烯酸酯 3。
-
Catalyst-free chemoselective reduction of the carbon–carbon double bond in conjugated alkenes with Hantzsch esters in water作者:Qi He、Zhihong Xu、Dehong Jiang、Wensi Ai、Ronghua Shi、Shan Qian、Zhouyu WangDOI:10.1039/c3ra48072k日期:——A simple, efficient and green protocol for chemoselective reduction of carbon–carbon double bond in conjugated alkenes with Hantzsch esters is described. Without any additional catalysts, a series of conjugated alkenes with strong electron-withdrawing groups were reduced in water with excellent yield. Functional groups such as nitrile, ester, nitro, fluoro, chloro, bromo, furanyl and benzyl are all
-
Rearrangement of Propargylic Esters: Metal-Based Stereospecific Synthesis of (<i>E</i>)- and (Z)-Knoevenagel Derivatives作者:José Barluenga、Lorena Riesgo、Rubén Vicente、Luis A. López、Miguel TomásDOI:10.1021/ja072864r日期:2007.6.1catalysts perform the isomerization with complementary Z/E selectivity. Moreover, alkynyl-conjugated Knoevenagel products are produced from (bisalkynyl)methyl acetates. In such a case, the reaction is chemoselective as the 1,3-acetyl migration takes place through the alkoxyalkyne group in preference over the phenylalkyne group. The resulting (E)-alkynylenone unit suffers metal-catalyzed cyclization
表征谱图
-
氢谱1HNMR
-
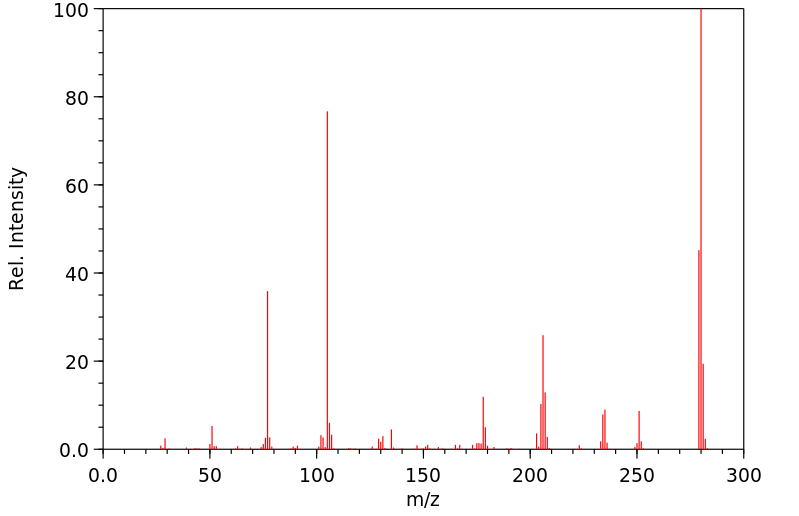
质谱MS
-
碳谱13CNMR
-
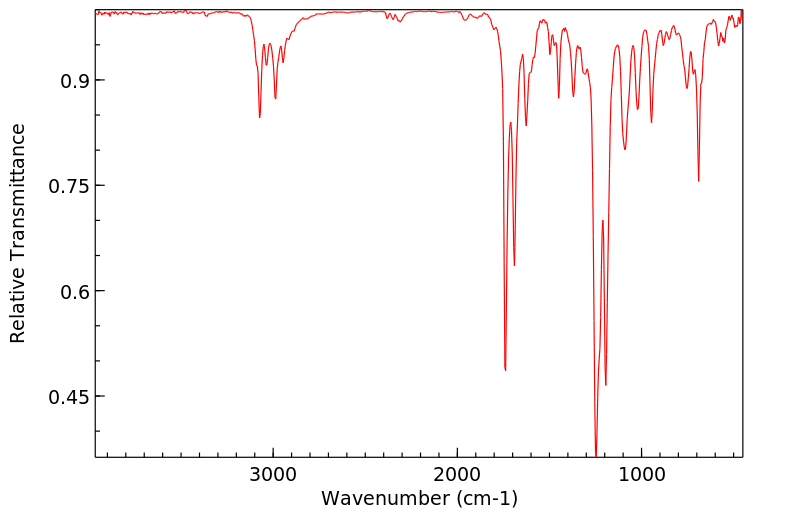
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2Z)-1,3-二苯基-2-丙烯-1-酮,2-丙烯-1-酮,1,3-二苯基-,(2Z)-
龙血素D
龙血素A
龙血素 B
黄色当归醇F
黄色当归醇B
黄腐醇; 黄腐酚
黄腐醇 D; 黄腐酚 D
黄腐酚B
黄腐酚
黄腐酚
黄卡瓦胡椒素 C
高紫柳查尔酮
阿普非农
阿司巴汀
阿伏苯宗
金鸡菊查耳酮
邻肉桂酰苯甲酸
达泊西汀杂质25
豆蔻明
补骨脂色烯查耳酮
补骨脂查耳酮
补骨脂呋喃查耳酮
补骨脂乙素
蜡菊亭; 4,2',4'-三羟基-6'-甲氧基查耳酮
苯酚,4-[3-(2-羟基苯基)-1-苯基丙基]-2-(3-苯基丙基)-
苯磺酰胺,N-[4-[3-(3-羟基苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,N-[3-[3-(4-羟基-3-甲氧苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,4-甲氧基-N,N-二甲基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯化,4,5-二甲氧基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯,4-甲氧基-3-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯甲醇,4-甲氧基-a-[2-(4-甲氧苯基)乙烯基]-
苯甲酸-[4-(3-氧代-3-苯基-丙烯基)-苯胺]
苯甲酸,3-[3-(4-溴苯基)-1-羰基-2-丙烯基]-4-羟基-
苯甲酰(2-羟基苯酰)甲烷
苯甲腈,4-(1-羟基-3-羰基-3-苯基丙基)-
苯基[2-(1-萘基)乙烯基]甲酮
苯基-(三苯基-丙-2-炔基)-醚
苯基-(2-苯基-2,3-二氢-苯并噻唑-2-基)-甲酮
苯亚甲基苯乙酮
苯乙酰腈,a-(1-氨基-2-苯基亚乙基)-
苯丙酸,a-苯甲酰-b-羰基-,苯基(苯基亚甲基)酰肼
苯,1-(2,2-二甲基-3-苯基丙基)-2-甲基-
苏木查耳酮
苄桂哌酯
苄基(4-氯-2-(3-氧代-1,3-二苯基丙基)苯基)氨基甲酸酯
芦荟提取物
腈苯唑
胀果甘草宁C
聚磷酸根皮酚