3'-bromo-2'-hydroxy-propiophenone | 17764-91-9
中文名称
——
中文别名
——
英文名称
3'-bromo-2'-hydroxy-propiophenone
英文别名
2-Brom-6-propionyl-phenol;Propiophenone, 3'-bromo-2'-hydroxy-;1-(3-bromo-2-hydroxyphenyl)propan-1-one
CAS
17764-91-9
化学式
C9H9BrO2
mdl
——
分子量
229.073
InChiKey
BGTYULWRWLOCQS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:55–56°C
-
沸点:268.9±25.0 °C(Predicted)
-
密度:1.506±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.22
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2'-羟基苯丙酮 1-(2-Hydroxy-phenyl)-propan-1-on 610-99-1 C9H10O2 150.177 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(3,5-二溴-2-羟基苯基)-1-丙酮 1-(3,5-dibromo-2-hydroxy-phenyl)-propan-1-one 2887-68-5 C9H8Br2O2 307.969 —— 2-Brom-6-<1-hydroxy-propyl>-anisol 23600-59-1 C10H13BrO2 245.116
反应信息
-
作为反应物:描述:参考文献:名称:Martin,R.; Betoux,J.M., Bulletin de la Societe Chimique de France, 1969, p. 2079 - 2088摘要:DOI:
-
作为产物:描述:参考文献:名称:Martin,R.; Betoux,J.M., Bulletin de la Societe Chimique de France, 1969, p. 2079 - 2088摘要:DOI:
文献信息
-
Synthesis of Tetrasubstituted Enamines through Borane-Catalyzed Hydrogenations作者:Zijia Zhang、Xiangqing Feng、Haifeng DuDOI:10.1021/acs.orglett.3c03578日期:2023.12.29This paper describes a B(C6F5)3-catalyzed hydrogenation of β-substituted α,β-unsaturated imines by using as low as 0.2 mol % catalyst. A variety of tetrasubstituted enamines were afforded in 95–99% yields. It provides an efficient and facile way without the need for column chromatography purification.本文描述了使用低至0.2 mol% 的催化剂进行B(C 6 F 5 ) 3催化的β-取代的α,β-不饱和亚胺的氢化反应。多种四取代烯胺的产率达到 95-99%。它提供了一种有效且简便的方法,无需柱色谱纯化。
-
Effect of ortho-hydroxy substituent on photochemistry of 2-bromo-1-phenylalkan-1-ones: Remote bromine atom shift reaction作者:Ho Suk Shin、Bong Ser ParkDOI:10.1016/j.tet.2023.133660日期:2023.10forms ring-brominated phenyl ketones, where bromine moves from the α-position of the carbonyl to the phenyl ring. The unprecedented remote Br shift reaction occurs with regioselectivity favoring the ortho isomer, which originates from a hypobromite intermediate. The reaction has several advantages over other known methods for the preparation of the ring-brominated 1-(2-hydroxy)phenylalkan-1-ones: no
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
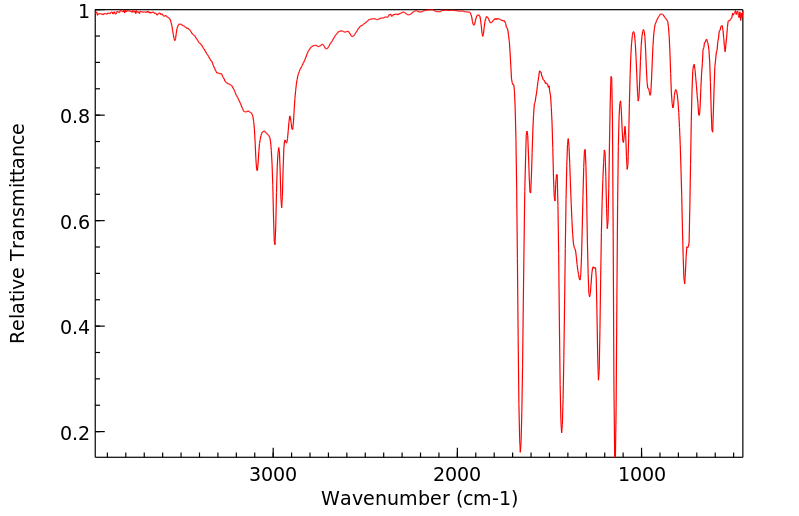
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷