(E)-4-(3-(4-methoxyphenyl)-3-oxoprop-1-enyl)benzonitrile
分子结构分类
中文名称
——
中文别名
——
英文名称
(E)-4-(3-(4-methoxyphenyl)-3-oxoprop-1-enyl)benzonitrile
英文别名
(E)-4-[3-(4-methoxyphenyl)-3-oxoprop-1-en-1-yl]benzonitrile;4-[(E)-3-(4-methoxyphenyl)-3-oxoprop-1-enyl]benzonitrile
CAS
——
化学式
C17H13NO2
mdl
——
分子量
263.296
InChiKey
WCCHVTMSZURADI-IZZDOVSWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:20
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.06
-
拓扑面积:50.1
-
氢给体数:0
-
氢受体数:3
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:WAGNER G.; VOIGT B., PHARMAZIE
, 1976, 31, NO 8, 528-532 摘要:DOI: -
作为产物:描述:4-溴苯腈 、 1'-羟基-2',3'-去氢草蒿脑 在 bis-triphenylphosphine-palladium(II) chloride copper(l) iodide 、 三乙胺 作用下, 以 四氢呋喃 为溶剂, 反应 0.25h, 生成 (E)-4-(3-(4-methoxyphenyl)-3-oxoprop-1-enyl)benzonitrile参考文献:名称:Microwave-Accelerated Coupling-Isomerization-Enamine Addition-Aldol Condensation Sequences to 1-Acetyl-2-amino-cyclohexa-1,3-dienes摘要:微波加速的连贯反应中,电子缺乏的(杂)芳基溴化物1、(杂)芳基丙炔醇2以及烯胺羰基化合物6或8,分别以良好的收率生成1-乙酰基-2-氨基环己-1,3-二烯7或6-N,N-二甲基氨基甲酰基环己烯酮9,这一过程可以看作是一个锅内耦合-异构化-烯胺加成-醛醇缩合的序列反应。DOI:10.1055/s-2006-947352
文献信息
-
Unexpected solvent/substitution-dependent inversion of the enantioselectivity in Michael addition reaction using chiral phase transfer catalysts作者:Ponmuthu Kottala Vijaya、Sepperumal Murugesan、Ayyanar SivaDOI:10.1016/j.tetlet.2015.07.074日期:2015.9New cinchonium salts bearing 5,5′-bis(methyl)-2,2′-bipyridine 1 group show solvent/substitution-dependent reversal of enantioselectivity. When used as chiral phase transfer catalyst in the asymmetric Michael addition of chalcones with diethylmalonate within two hours these catalysts result in high chemical yield (up to 98%) and enantiomeric excess (up to 99%) under lower concentrations of base and cold
-
[EN] NUCLEAR RECEPTOR MODULATORS AND THEIR USE FOR THE TREATMENT AND PREVENTION OF CANCER<br/>[FR] MODULATEURS DE RÉCEPTEURS NUCLÉAIRES ET LEUR UTILISATION POUR LE TRAITEMENT ET LA PRÉVENTION D'UN CANCER申请人:US HEALTH公开号:WO2012174436A1公开(公告)日:2012-12-20Disclosed are compounds which are nuclear receptor modulators that can act as antagonists to the androgen receptor, for example, a compound of Formula I: wherein R1 to R5 and X1 to X5 are as described herein, as well as pharmaceutically acceptable salts, solvates, and stereoisomers thereof. Pharmaceutical compositions comprising such compounds, as well as methods of use, and treatment for cancers, including prostate cancers, other nuclear receptor mediated cancers, and other conditions, are also disclosed.本文披露了一些化合物,它们是核受体调节剂,可以作为雄激素受体的拮抗剂,例如,公式I的化合物:其中R1至R5和X1至X5如本文所述,以及其药用盐、溶剂化合物和立体异构体。还披露了包括这些化合物的药物组合物,以及使用方法和治疗癌症,包括前列腺癌、其他核受体介导的癌症和其他疾病的方法。
-
Antimalarial Alkoxylated and Hydroxylated Chalones: Structure−Activity Relationship Analysis作者:Mei Liu、Prapon Wilairat、Mei-Lin GoDOI:10.1021/jm0101747日期:2001.12.1among the active compounds. Hydroxylated chalcones were less active than the corresponding alkoxylated analogues. When evaluated in vivo, 8 and 208 were comparable to chloroquine in extending the lifespan of infected mice. Multivariate data analysis showed that in vitro activity was mainly determined by the properties of ring B. Quantitative structure-activity relationship models with satisfactory predictive合成在环B上具有2',3',4'-三甲氧基,2',4'-二甲氧基,4'-甲氧基,4'-乙氧基,2',4'-二羟基和4'-羟基的邻苯二甲酰并在[3H]次黄嘌呤摄取测定中针对恶性疟原虫(K1)进行了体外评估。另一个环A是具有不同亲脂性的给电子或吸电子取代基的喹啉,吡啶,萘或苯环。三甲氧基6和27,二甲氧基7,8,29和甲氧基31类似物具有良好的体外活性(IC(50)<5 microM)。在活性化合物中很好地代表了3-喹啉基环A衍生物。羟基查耳酮的活性低于相应的烷氧基化类似物。在体内评估时,8和208在延长被感染小鼠的寿命方面与氯喹相当。多变量数据分析表明,体外活性主要取决于环B的特性。使用对潜在结构的投影,获得了各种B环查耳酮具有令人满意的预测能力的定量构效关系模型。提出了一个具有良好可预测性的模型,用于19个活动查尔肯。尺寸和疏水性被确定为关键参数。
-
Photoredox-Catalyzed Cyclopropanation of Michael Acceptors作者:Ana M. del Hoyo、Marcos García SueroDOI:10.1002/ejoc.201601604日期:2017.4.18A new protocol for the catalytic cyclopropanation of α,β-unsaturated carbonyl compounds with diiodomethane by means of photoredox catalysis has been successfully developed. The transformation is characterized by its mild conditions, functional group compatibility, and excellent selectivity profile.
-
A facile approach to highly functional trisubstituted furans via intramolecular Wittig reactions作者:Ko-Wei Chen、Siang-en Syu、Yeong-Jiunn Jang、Wenwei LinDOI:10.1039/c0ob00912a日期:——An efficient and mild synthesis of trisubstituted furans, starting from α,β-unsaturated ketones, tributylphosphine, and acyl chlorides, is described. The strategy employs the intramolecular Wittig protocol as a key step to install the crucial furan ring, leading to a wide variety of highly functional furans in one step.
表征谱图
-
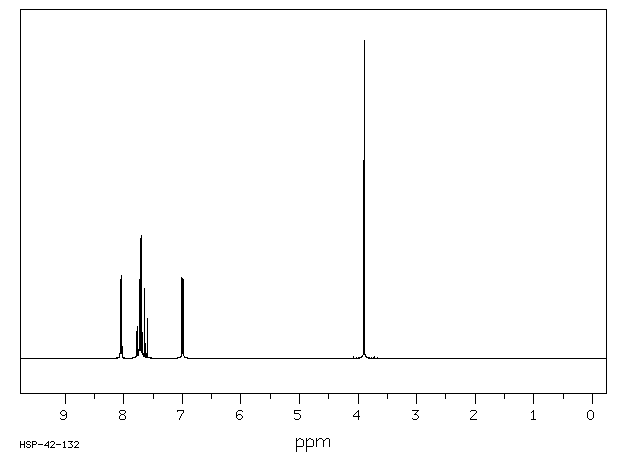
氢谱1HNMR
-
质谱MS
-
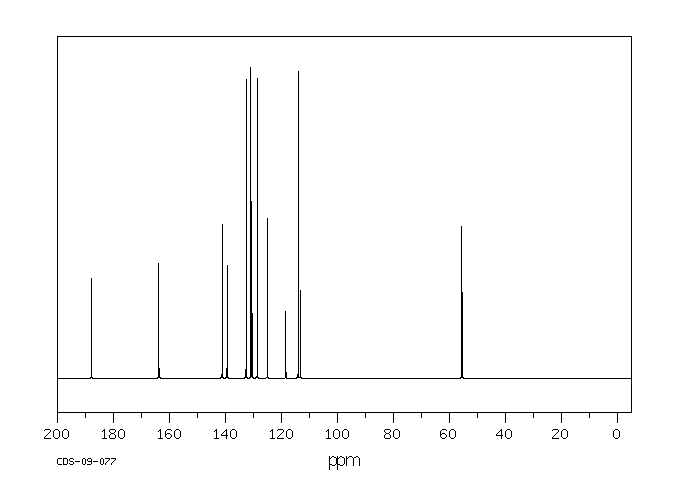
碳谱13CNMR
-
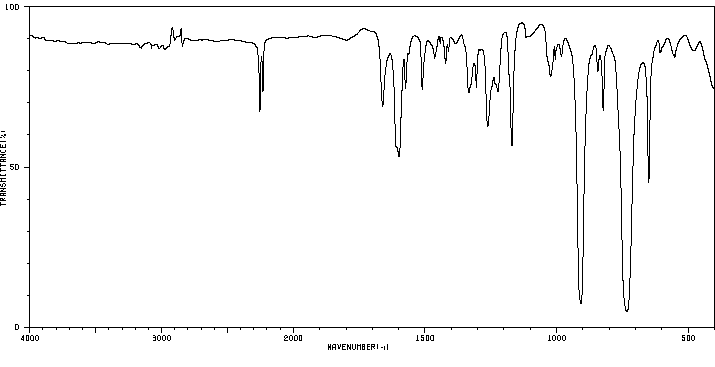
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2Z)-1,3-二苯基-2-丙烯-1-酮,2-丙烯-1-酮,1,3-二苯基-,(2Z)-
龙血素D
龙血素A
龙血素 B
黄色当归醇F
黄色当归醇B
黄腐醇; 黄腐酚
黄腐醇 D; 黄腐酚 D
黄腐酚B
黄腐酚
黄腐酚
黄卡瓦胡椒素 C
高紫柳查尔酮
阿普非农
阿司巴汀
阿伏苯宗
金鸡菊查耳酮
邻肉桂酰苯甲酸
达泊西汀杂质25
豆蔻明
补骨脂色烯查耳酮
补骨脂查耳酮
补骨脂呋喃查耳酮
补骨脂乙素
蜡菊亭; 4,2',4'-三羟基-6'-甲氧基查耳酮
苯酚,4-[3-(2-羟基苯基)-1-苯基丙基]-2-(3-苯基丙基)-
苯磺酰胺,N-[4-[3-(3-羟基苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,N-[3-[3-(4-羟基-3-甲氧苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,4-甲氧基-N,N-二甲基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯化,4,5-二甲氧基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯,4-甲氧基-3-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯甲醇,4-甲氧基-a-[2-(4-甲氧苯基)乙烯基]-
苯甲酸-[4-(3-氧代-3-苯基-丙烯基)-苯胺]
苯甲酸,3-[3-(4-溴苯基)-1-羰基-2-丙烯基]-4-羟基-
苯甲酰(2-羟基苯酰)甲烷
苯甲腈,4-(1-羟基-3-羰基-3-苯基丙基)-
苯基[2-(1-萘基)乙烯基]甲酮
苯基-(三苯基-丙-2-炔基)-醚
苯基-(2-苯基-2,3-二氢-苯并噻唑-2-基)-甲酮
苯亚甲基苯乙酮
苯乙酰腈,a-(1-氨基-2-苯基亚乙基)-
苯丙酸,a-苯甲酰-b-羰基-,苯基(苯基亚甲基)酰肼
苯,1-(2,2-二甲基-3-苯基丙基)-2-甲基-
苏木查耳酮
苄桂哌酯
苄基(4-氯-2-(3-氧代-1,3-二苯基丙基)苯基)氨基甲酸酯
芦荟提取物
腈苯唑
胀果甘草宁C
聚磷酸根皮酚