2-巯基丙酸甲酯 | 53907-46-3
中文名称
2-巯基丙酸甲酯
中文别名
——
英文名称
methyl 2-mercaptopropionate
英文别名
methyl thiolactate;methyl 2-sulfanylpropanoate
CAS
53907-46-3
化学式
C4H8O2S
mdl
MFCD16041849
分子量
120.172
InChiKey
SNWKNPMDQONHKK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:69-70℃ (45 Torr)
-
密度:1.068±0.06 g/cm3(Predicted)
-
LogP:0.978 (est)
-
保留指数:813
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:27.3
-
氢给体数:1
-
氢受体数:3
安全信息
-
海关编码:2930909090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Chain transfer agents for raft polymerization in aqueous media摘要:通过产生分子量范围较窄的方式合成的聚合物和共聚物可能具有与传统方式合成的聚合物不同的性质。然而,为了获得这种聚合物,必须控制聚合过程。一种控制聚合的类型是可逆加成-断裂链转移(RAFT)过程,具有类似活性聚合的特征。本发明揭示了一组二硫酸酯和三硫酸酯,适用作为RAFT聚合的链转移剂。本发明还揭示了在水性介质中进行的RAFT聚合过程。公开号:US06855840B2
-
作为产物:参考文献:名称:中性铼(I)三羰基与硫供体配体的配合物:抗增殖活性和细胞定位摘要:铼( I )三羰基配合物因其细胞成像特性以及抗癌和抗微生物活性而被广泛研究,但与S-供体配体的配合物仍然相对未经探索。一系列六个fac -[Re(NN)(CO) 3 (SR)] 配合物,其中 (NN) 是 2,2′-联吡啶 (bipy) 或 1,10-菲咯啉 (phen),RSH 是一系列硫代羧酸甲酯的合成和表征。这些复合物在人乳腺癌细胞系(MDA-MB-231 和 MCF-7)中的细胞摄取和抗增殖活性通常低于先前描述的fac -[Re(NN)(CO) 3 (OH 2) )] +复合物;然而,其中一种复合物fac -[Re(CO) 3 (phen)(SC(Ph)CH 2 C(O)OMe)] ( 3b ) 在 72 小时处理时具有活性(IC 50 ∼ 10 μM)硫醇耗尽的 MDA-MB-231 细胞。此外,与fac -[Re(CO) 3 (phen)(OH 2 )] +不同,该复合物在细胞DOI:10.1039/d4dt00149d
文献信息
-
Highly Stereoselective Synthesis of the Anti-Platelet Activating Factor, 4-Thiazolidinones, Using Silyl Derivatives of 2-Mercaptoalkanoic Acids作者:Yoo Tanabe、Hitomi Okumura、Masaki Nagaosa、Masanari MurakamiDOI:10.1246/bcsj.68.1467日期:1995.5factor-active drugs. The use of the piperidine catalyst and no catalyst showed very high cis-stereoselectivity (cis/trans = 10/1—50/1) during the reaction. On the other hand, the trans-selective reaction was promoted by Ti(O-i-Bu)4 and Al(O-s-Bu)3 catalysts (cis/trans = 1/8—1/25). Both reactions were conducted with higher cis- and trans-selectivities as compared with those of the alkyl 2-mercaptoalkanoates under
-
Synthesis and spectroscopic studies of some hydrogenated thiazolo[2,3- a ]isoquinolines作者:Maria D Rozwadowska、Agnieszka SulimaDOI:10.1016/s0040-4020(01)00224-1日期:2001.44-dihydroisoquinoline derivatives under the action of α-mercapto alkanoic acids or ethylene sulfide, respectively. In the synthesis of compounds 2 and 5 isothiocarbostril (13) and N-thioacetyl-β-phenylethylamine derivatives (14), respectively, were also used as substrates and treated with bromoacetic acid derivatives. Spectral characteristics (IR, 1H, 13C NMR and MS) of compounds 1–12 are presented.
-
Stereoselective synthesis of anti-PAF active thiazolidin-4-ones via cyclo-condensation of alkyl α-mercaptocarboxylates with arylimines作者:Yoo Tanabe、Yoshi-no Kubota、Yuzuru Sanemitsu、Nobushige Itaya、Gohfu SuzukamoDOI:10.1016/s0040-4039(00)92634-0日期:1991.1Two distinctive methods for the synthesis of cis- and trans-2, 5-disubstituted-thiazolidin-4-ones via stereoselective cyclo-condensation between α-mercaptocarboxylic esters and arylimines have been developed. With the new reaction used as the key step, two sets of optically active anti-PAF active thiazolidin-4-ones were synthesized.
-
一种组合物及其应用申请人:广州同隽医药科技有限公司公开号:CN109970609B公开(公告)日:2021-05-11
-
Discovery of <i>N</i>-Phenylaminomethylthioacetylpyrimidine-2,4-diones as Protoporphyrinogen IX Oxidase Inhibitors through a Reaction Intermediate Derivation Approach作者:Da-Wei Wang、Lu Liang、Zhi-Yuan Xue、Shu-Yi Yu、Rui-Bo Zhang、Xia Wang、Han Xu、Xin Wen、Zhen XiDOI:10.1021/acs.jafc.1c00796日期:2021.4.14(NtPPO) inhibitory and herbicidal activity evaluations led to identifying some compounds with improved NtPPO inhibition potency than saflufenacil and good post-emergence herbicidal activity at 37.5–150 g of ai/ha. Among these analogues, ethyl 2-((((2-chloro-4-fluoro-5-(3-methyl-2,6-dioxo-4-(trifluoromethyl)-3,6-dihydropyrimidin-1(2H)-yl)phenyl)amino)methyl)thio)acetate (2c) (Ki = 11 nM), exhibited excellent原卟啉原氧化酶(PPO,EC 1.3.3.4)是发现绿色除草剂的有效靶点。在这项工作中,我们在研究我们最初设计的N-苯基尿嘧啶噻唑烷酮 ( 1 ) 的反应中间体的基础上,意外发现了一系列新的N-苯基氨基甲硫基乙酰基嘧啶-2,4-二酮 ( 2-6 ) 作为有前景的 PPO 抑制剂。开发了一种高效的一锅法程序,可以以良好到高的收率产生 41 种目标化合物。系统性烟草PPO (NtPPO) 抑制和除草活性评估导致鉴定出一些化合物,其 NtPPO 抑制效力优于苯嘧磺草胺,并且在 37.5-150 g ai/ha 下具有良好的芽后除草活性。在这些类似物中,乙基 2-((((2-氯-4-氟-5-(3-甲基-2,6-二氧代-4-(三氟甲基)-3,6-二氢嘧啶-1(2 H )-基)苯基)氨基)甲基)硫代)乙酸酯 ( 2c ) ( K i = 11 nM),在 37.5–150 g ai/ha 时表现出优异的杂草控制效果,并且在
表征谱图
-
氢谱1HNMR
-
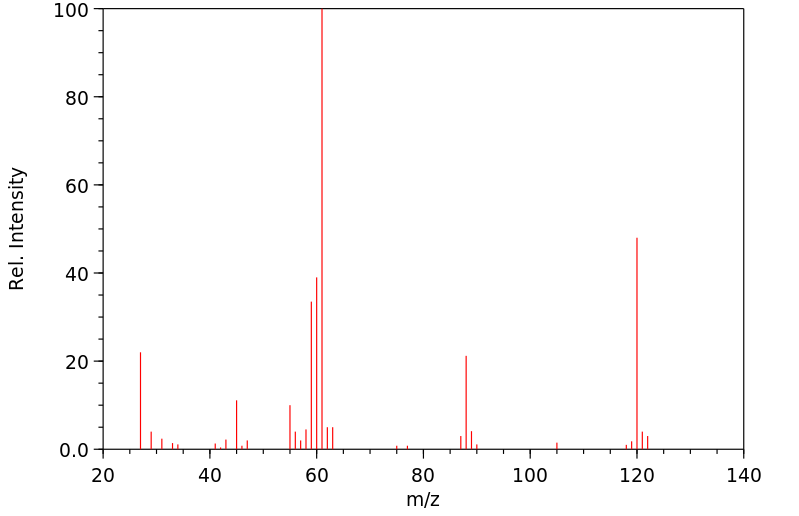
质谱MS
-
碳谱13CNMR
-
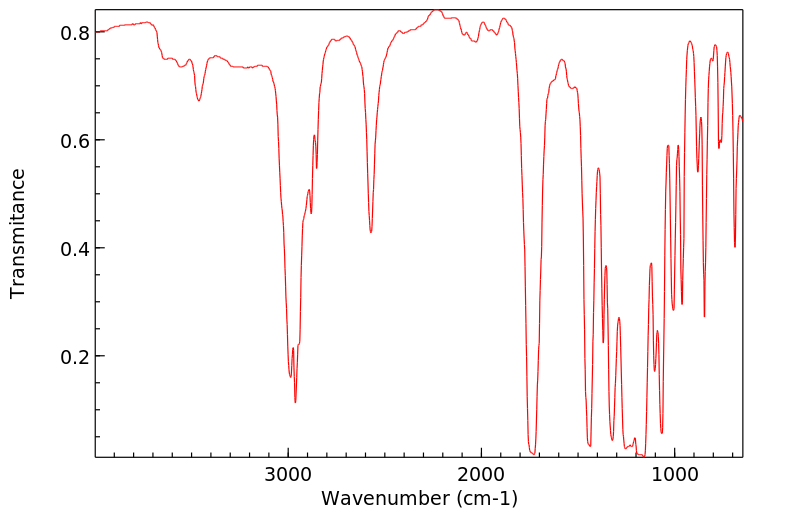
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸