2-异丁基乙酰乙酸乙酯 | 1522-34-5
中文名称
2-异丁基乙酰乙酸乙酯
中文别名
——
英文名称
ethyl 2-acetyl-4-methylpentanoate
英文别名
2-acetyl-4-methylpentanoic acid ethyl ester;α-Isobutyl-acetessigsaeure-ethylester;2-Isobutyl-acetessigsaeure-ethylester
CAS
1522-34-5
化学式
C10H18O3
mdl
MFCD00457371
分子量
186.251
InChiKey
ZGULNKYAODXSCS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:13
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2918300090
-
危险性防范说明:P305+P351+P338
-
危险性描述:H227,H315,H319,H335
-
储存条件:室温
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl 2-bromoacetyl-4-methylpentanoate —— C10H17BrO3 265.147 2-乙酰基-4-甲基-戊酸 2-acetyl-4-methyl-pentanoic acid 5699-53-6 C8H14O3 158.197 —— ethyl (R)-2-[(S)-1-hydroxyethyl]-4-methylpentanoate 1370590-68-3 C10H20O3 188.267
反应信息
-
作为反应物:描述:参考文献:名称:Takizawa, 1952, vol. 4, p. 311摘要:DOI:
-
作为产物:描述:参考文献:名称:Suryawanshi, S N; Rani, A; Bhakuni, D S, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1991, vol. 30, # 12, p. 1089 - 1094摘要:DOI:
文献信息
-
Novel compounds and compositions as protease inhibitors申请人:——公开号:US20020052378A1公开(公告)日:2002-05-02The present invention relates to novel cysteine protease inhibitors of Formula I: 1 the pharmaceutically acceptable salts and N-oxide derivatives thereof, their use as therapeutic agents and methods of making them.
-
Rh(I)–Bisphosphine-Catalyzed Asymmetric, Intermolecular Hydroheteroarylation of α-Substituted Acrylate Derivatives作者:Claire M. Filloux、Tomislav RovisDOI:10.1021/ja511445x日期:2015.1.14Asymmetric hydroheteroarylation of alkenes represents a convenient entry to elaborated heterocyclic motifs. While chiral acids are known to mediate asymmetric addition of electron-rich heteroarenes to Michael acceptors, very few methods exploit transition metals to catalyze alkylation of heterocycles with olefins via a C–H activation, migratory insertion sequence. Herein, we describe the development烯烃的不对称氢杂芳基化代表了复杂杂环基序的便捷进入。虽然已知手性酸可以介导富电子杂芳烃向 Michael 受体的不对称加成,但很少有方法利用过渡金属通过 C-H 活化、迁移插入序列催化杂环与烯烃的烷基化。在此,我们描述了α-取代丙烯酸酯与苯并恶唑的不对称分子间氢杂芳基化反应的发展。该反应以中等至优异的产率和良好至优异的对映选择性提供2-取代的苯并恶唑。值得注意的是,一系列机制研究似乎与涉及 Rh(I)-烯醇化物对映选择性质子化的途径相矛盾,尽管事实上在芳基硼酸与甲基丙烯酸酯衍生物的相关加成中几乎一致地调用了这种机制。相反,有证据表明,迁移插入或β-氢化物消除具有对映决定性,Rh(I)-烯醇化物异构化为Rh(I)-杂苄基物质可将所得的α-立构中心与差向异构化隔离开来。庞大的配体 CTH-(R)-Xylyl-P-Phos 对于反应性和对映选择性至关重要,因为它可能会阻止苯并恶唑底物或中间体与循
-
Combined Thallium-201 and Dynamic Iodine-123 Iodophenylpentadecanoic Acid Single-Photon Emission Computed Tomography in Patients after Acute Myocardial Infarction with Effective Reperfusion作者:Wolf-S. Richter、Michael Cordes、Torsten Schuppenhauer、Dieter L. Munz、Stephan Beckmann、Michael SchartlDOI:10.1002/clc.4960231210日期:2000.12expected during ischemia and reperfusion. In ischemic myocardium, the oxidative degradation of carbohydrates is shifted toward the anaerobic production of lactate and the oxidation of fatty acids is suppressed. HYPOTHESIS The aim of this study was to examine the uptake and metabolism of iodine-123 (123I) iodophenylpentadecanoic acid (IPPA) in stunned myocardium. METHODS In 15 patients, SPECT with 201Tl and背景技术在缺血和再灌注期间,预期能量代谢的显着紊乱。在缺血性心肌中,碳水化合物的氧化降解向乳酸的厌氧产生转移,并且抑制了脂肪酸的氧化。假设这项研究的目的是检查震惊的心肌中碘123(123I)碘苯基十五碳二烯酸(IPPA)的吸收和代谢。方法在15例患者中,在心肌梗死再灌注后12 +/- 5天进行SPECT联合201T1和123I IPPA以及低剂量多巴酚丁胺刺激的超声心动图检查。在24 +/- 8天后进行随访超声心动图检查,以记录功能改善情况。在五个左心室节段中摄取201T1和123I IPPA,动态SPECT成像用于计算双指数心肌123I IPPA清除的快和慢成分。结果26个功能失调的部位中有14个部位的壁运动得到了改善(54%)。震惊的节段的特征在于减少的123I IPPA提取,快速的半衰期较短,慢速清除组分的半衰期较长。组合的201T1 / 123I IPPA研究的所有参数都预测了具有
-
Theoretical investigation of the reaction of dialkylzincs with α-alkoxycarbonyl radicals. Evaluation of α-bromoacrylates as radical acceptors in radical-polar crossover processes作者:François Vibert、Julien Maury、Hugo Lingua、Eric Besson、Didier Siri、Michèle P. Bertrand、Laurence FerayDOI:10.1016/j.tet.2015.09.045日期:2015.11The evaluation of ethyl α-bromoacrylate as radical acceptor in dialkylzinc radical-polar cascades addresses the question of the parameters controlling homolytic substitution at zinc by α-alcoxycarbonyl radicals. Under non-degassed medium ethyl α-bromoacrylate reacts with diethylzinc to give a bromocyclopropane. The reaction involves successively radical addition, SH2 at zinc, conjugate addition of
-
Klebsiellapneumoniae (NBRC 3319) Mediated Asymmetric Reduction of α-Substituted β-Oxo Esters and Its Application to the Enantioiselective Synthesis of Small-Ring Carbocycle Derivatives作者:Rajib Bhuniya、Tridib Mahapatra、Samik NandaDOI:10.1002/ejoc.201101695日期:2012.3Ketoreductases from Klebsiella pneumoniae (NBRC 3319) selectively reduce several 2-substituted ethyl 3-oxobutyrates to yield the corresponding syn-β-hydroxy esters with remarkable stereocontrol (de > 99 %, ee > 99 %). The enantiopure hydroxy oxo esters were synthetically manipulated to access new small-ring carbocycles.
表征谱图
-
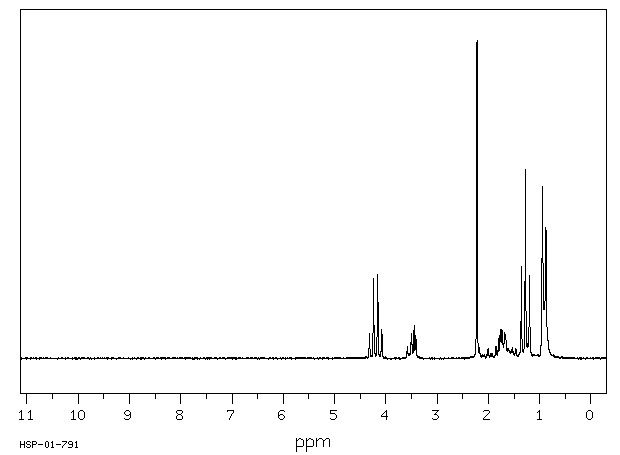
氢谱1HNMR
-
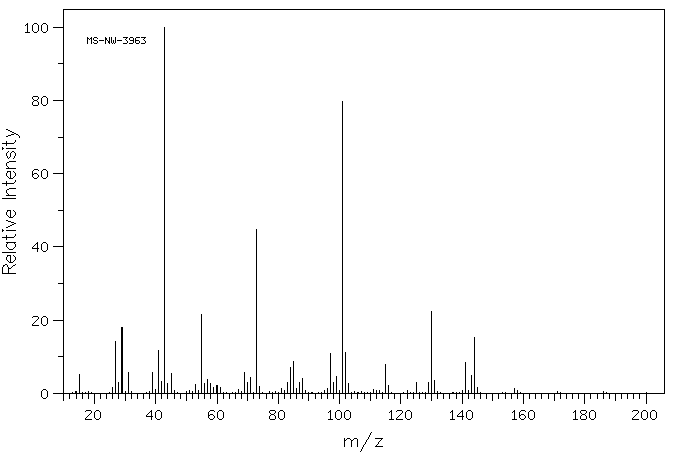
质谱MS
-
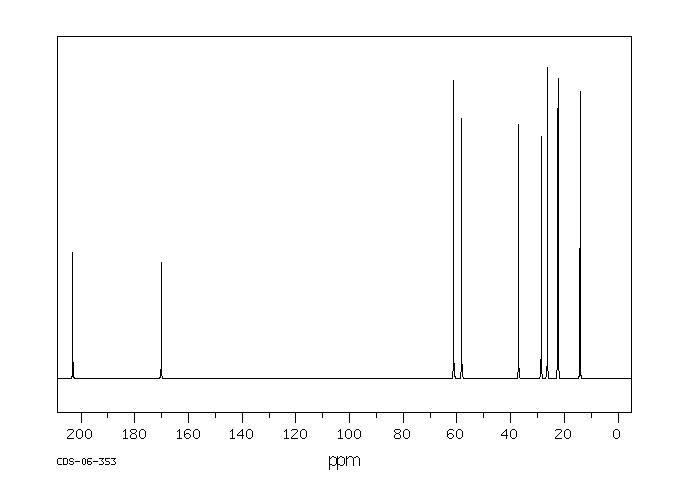
碳谱13CNMR
-
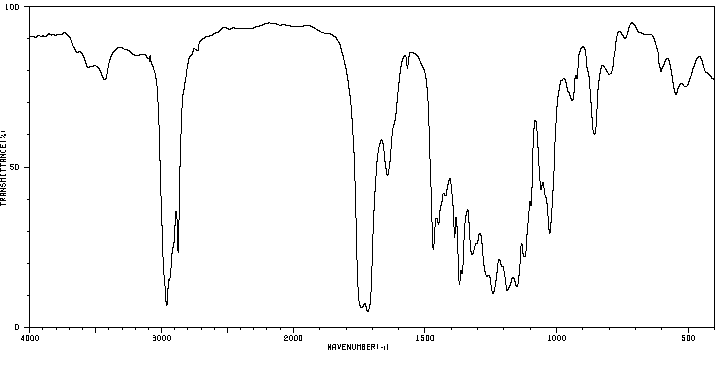
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰基乙酸
顺-3-己烯-1-丙酮酸
青霉酸
钠氟草酰乙酸二乙酯
醚化物
酮霉素
辛酸,2,4-二羰基-,乙基酯
草酸乙酯钠盐
草酰乙酸二乙酯钠盐
草酰乙酸二乙酯
草酰乙酸
草酰丙酸二乙酯
苯乙酰丙二酸二乙酯
苯丁酸,b-羰基-,2-丙烯基酯
聚氧化乙烯
羟基-(3-羟基-2,3-二氧代丙基)-氧代鏻
磷酸二氢2-{(E)-2-[4-(二乙胺基)-2-甲基苯基]乙烯基}-1,3,3-三甲基-3H-吲哚正离子
碘化镝
硬脂酰乙酸乙酯
甲氧基乙酸乙酯
甲氧基乙酰乙酸酯
甲基氧代琥珀酸二甲盐
甲基4-环己基-3-氧代丁酸酯
甲基4-氯-3-氧代戊酸酯
甲基4-氧代癸酸酯
甲基4-氧代月桂酸酯
甲基4-(甲氧基-甲基磷酰)-2,2,4-三甲基-3-氧代戊酸酯
甲基3-羰基-2-丙酰戊酸酯
甲基3-氧代十五烷酸酯
甲基2-氟-3-氧戊酯
甲基2-氟-3-氧代己酸酯
甲基2-氟-3-氧代丁酸酯
甲基2-乙酰基环丙烷羧酸酯
甲基2-乙酰基-4-甲基-4-戊烯酸酯
甲基2-乙酰基-2-丙-2-烯基戊-4-烯酸酯
甲基2,5-二氟-3-氧代戊酸酯
甲基2,4-二氟-3-氧代戊酸酯
甲基2,4-二氟-3-氧代丁酸酯
甲基1-异丁酰基环戊烷羧酸酯
甲基1-乙酰基环戊烷羧酸酯
甲基1-乙酰基环丙烷羧酸酯
甲基1-乙酰基-2-乙基环丙烷羧酸酯
甲基(2Z,4E,6E)-2-乙酰基-7-(二甲基氨基)-2,4,6-庚三烯酸酯
甲基(2S)-2-甲基-4-氧代戊酸酯
甲基(1S,2R)-2-乙酰基环丙烷羧酸酯
甲基(1R,2R)-2-乙酰基环丙烷羧酸酯
瑞舒伐他汀杂质
瑞舒伐他汀杂质
环氧乙烷基甲基乙酰乙酸酯
环戊戊烯酸,Β-氧代,乙酯