2-氨基-5-溴-2'-氟二苯甲酮 | 1479-58-9
中文名称
2-氨基-5-溴-2'-氟二苯甲酮
中文别名
2-氨基-5-溴-2’-氟二苯甲酮;2-氨基-5-溴-2"-氟二苯甲酮
英文名称
2-amino-5-bromo-2′-fluorobenzophenone
英文别名
(2-amino-5-bromophenyl)(2-fluorophenyl)methanone;2-amino-5-bromo-2'-fluorobenzophenone;2-Amino-5-brom-2'-fluor-benzophenon;2-amino-5-bromo-2’-fluorobenzophenone;5-bromo-2'-fluoro-2-aminobenzophenone;(2-amino-5-bromophenyl)-(2-fluorophenyl)methanone
CAS
1479-58-9
化学式
C13H9BrFNO
mdl
MFCD00038380
分子量
294.123
InChiKey
XCOKDXNGCQXFCV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:100-104 °C
-
沸点:450.9±45.0 °C(Predicted)
-
密度:1.5259 (estimate)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
保留指数:2079;2090
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:43.1
-
氢给体数:1
-
氢受体数:3
安全信息
-
安全说明:S24/25
-
海关编码:2922399090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封保存,宜存放在阴凉、干燥的仓库中。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
2-Amino-5-bromo-2’-fluorobenzophenone
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
2-Amino-5-bromo-2’-fluorobenzophenone
Ingredient name:
CAS number: 1479-58-9
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C13H9BrFNO
Molecular weight: 294.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen fluoride, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
2-Amino-5-bromo-2’-fluorobenzophenone
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
2-Amino-5-bromo-2’-fluorobenzophenone
Ingredient name:
CAS number: 1479-58-9
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C13H9BrFNO
Molecular weight: 294.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen fluoride, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
2-氨基-5-溴-2'-氟二苯甲酮是一种用于有机合成中间体和医药中间体的重要化合物,主要应用于实验室的研发过程及化工生产的各个环节。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-2-氟苯甲酮 2-amino-2'-fluorobenzophenone 1581-13-1 C13H10FNO 215.227 —— 2-Bromacetamino-5-brom-2'-fluor-benzophenon 1647-74-1 C15H10Br2FNO2 415.056 —— (S)-{1-[4-bromo-2-(2-fluoro-benzoyl)-phenylcarbamoyl]-ethyl}-carbamic acid tert-butyl ester —— C21H22BrFN2O4 465.319 —— tert-butyl (R)-(1-((4-bromo-2-(2-fluorobenzoyl)-phenyl)amino)-1-oxopropan-2-yl)carbamate —— C21H22BrFN2O4 465.319
反应信息
-
作为反应物:描述:2-氨基-5-溴-2'-氟二苯甲酮 在 盐酸 、 N,N'-二环己基碳二亚胺 、 sodium hydroxide 作用下, 以 1,4-二氧六环 、 甲醇 、 二氯甲烷 、 水 为溶剂, 反应 77.25h, 生成 (R)-7-bromo-5-(2-fluorophenyl)-3-methyl-1,3-dihydro-2H-benzo[e][1,4]diazepin-2-one参考文献:名称:改进的临床哮喘候选药物 MIDD0301 的放大合成和纯化。摘要:我们报告了 MIDD0301 的改进和可扩展合成,这是一种正 GABA A受体调节剂,正在开发用于哮喘的口服和吸入治疗。与临床使用的其他苯二氮卓类药物相比,MIDD0301 是一种脑吸收有限的手性化合物。生成 MIDD0301 的起始材料是 2-amino-5-bromo-2'-fluoro二苯甲酮,由于邻位和对位的吸电子取代基,它具有非碱性氮位置,降低其对活化羧酸的反应性。由于转化不完全,对多克规模的肽偶联试剂的研究导致中等产量。其次,用于形成七元 1,4-二氮杂环的基本条件导致手性中心的外消旋化。我们发现与伯胺的 p K a相当的中性条件足以支持分子内亚胺的形成,但不能同时去除保护基团。通过应用d的N-羧酸酐克服了这两个困难-丙氨酸。在酸存在下活化,该化合物与非碱性 2-氨基-5-溴-2'-氟二苯甲酮反应并在与三乙胺中和后形成 1,4-二氮杂。精心设计的后处理程序和合成中间体在溶剂和溶DOI:10.1021/acs.oprd.0c00200
-
作为产物:描述:5-溴靛红 在 N-甲基吗啉 、 正丁基锂 、 双氧水 、 O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate 、 sodium hydroxide 作用下, 以 四氢呋喃 、 正己烷 、 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 1.5h, 生成 2-氨基-5-溴-2'-氟二苯甲酮参考文献:名称:氟溴西epa位置异构体的合成用于法医分析。摘要:最近,在许多法医案件中,设计师苯二氮卓类药物已作为联邦定点药物的合法替代品出现,如地西epa(Valium)和阿普唑仑(Xanax)。尽管当前的取证工具技术通常足以识别新型精神活性物质,但它们可能不容易区分潜在的位置异构体。另外,已知设计者苯并二氮杂s的位置异构体的表征数据广泛不存在。在这项研究中,氟溴马西m是自2012年以来公认的苯并二氮杂designer设计者,由于其有可能在美国进行联邦检定和目前的法律地位,因此被用于合成和表征。开发了一种实用的合成方法,以制备氟溴西m的每个位置异构体的纯化参考物质,其中溴和氟取代基的位置不同。成功制备了可能的异构体(12种中的9种),并用于进一步分析。DOI:10.1021/acs.joc.9b01433
文献信息
-
Solvent and Base in One: Tetra-<i>n</i> -butylammonium Acetate as a Multi-Purpose Ionic Liquid Medium for Ru-Catalyzed Directed Mono- and Di-<i>o</i> -C-H Arylation Reactions作者:Ya-Ling Zou、Zhen-Yu Wang、Yin-Mao Feng、You-Gui Li、Eric Assen B. KantchevDOI:10.1002/ejoc.201701022日期:2017.11.16Bu4NOAc can be a multi-purpose medium for a Ru-catalyzed directed o-C–H arylation reaction with functionalized aryl bromides. Acetate plays the role of base and Bu4N salts (excess acetate and side product bromide) behave as ionic liquids. The medium is compatible with both RuCl3·xH2O (major product: di-arylation) and [RuCl2(p-cymene)]2/(p-Tol)3P catalytic systems (major product: mono-arylation).
-
Metal‐Free Synthesis of Anthranils by PhIO Mediated Heterocyclization of <i>ortho</i> ‐Carbonyl Anilines作者:Alankrita Garia、Jatin Grover、Nidhi JainDOI:10.1002/ejoc.202100756日期:2021.8.6A metal-free synthesis of anthranils from ortho-carbonyl anilines using PhIO as a sole reagent under ambient conditions is described. No external additives are required, the reaction has broad substrate scope and delivers anthranils in excellent yields via oxidative heterocyclization.
-
Cleavage of C–C Bonds for the Synthesis of C2-Substituted Quinolines and Indoles by Catalyst-Controlled Tandem Annulation of 2-Vinylanilines and Alkynoates作者:Jixiang Ni、Yong Jiang、Zhenyu An、Rulong YanDOI:10.1021/acs.orglett.8b00260日期:2018.3.16and alkynoates through C–C bond cleavage is developed. With these general methods, 2-substituted indoles and quinolines can be accessed via tandem Michael addition and cyclization with no requirement of oxidant. This strategy not only provides a method for the synthesis C2-substituted indoles in good yields through the simultaneous cleavage of C═C and C≡C bonds under metal-free conditions but also provides
-
tert-Butyl nitrite (TBN) as the N atom source for the synthesis of substituted cinnolines with 2-vinylanilines and a relevant mechanism was studied作者:XiaoBo Pang、LianBiao Zhao、DaGang Zhou、Ping Yong He、ZhenYu An、Ji Xiang Ni、RuLong YanDOI:10.1039/c7ob01553d日期:——A green method to synthesize cinnolines by 6π electrocyclic reaction with alkenyl amines and TBN has been developed. TBN plays a dual role both as the nitrogen atom source and oxidant in this procedure. Relevant mechanism experiments reveal the reaction proceeds through electrocyclic reaction and with diazo hydroxide as a key intermediate.已开发出一种绿色方法,可通过与烯基胺和TBN的6π环化反应合成辛啉。TBN在此过程中既充当氮原子源又充当氧化剂。相关的机理实验表明,反应是通过环化反应进行的,并以重氮氢氧化物为关键中间体。
-
Copper-Catalyzed Tandem Aerobic Oxidative Cyclization for the Synthesis of Polysubstituted Quinolines via C(sp<sup>3</sup>)/C(sp<sup>2</sup>)–H Bond Functionalization作者:Xiaobo Pang、Mingzhong Wu、Jixiang Ni、Fuming Zhang、Jingfeng Lan、Baohua Chen、Rulong YanDOI:10.1021/acs.joc.7b01575日期:2017.10.6One-pot Cu-catalyzed tandem aerobic oxidative cyclization for the synthesis of quinolines from 2-vinylanilines/2-arylanilines and 2-methylquinolines via C(sp3)–H/C(sp2)–H bond functionalization has been developed. Dioxygen as an ideal oxidant has been employed for this transformation. The substrates bearing various functional groups perform well in this process and generate the desired products in
表征谱图
-
氢谱1HNMR
-
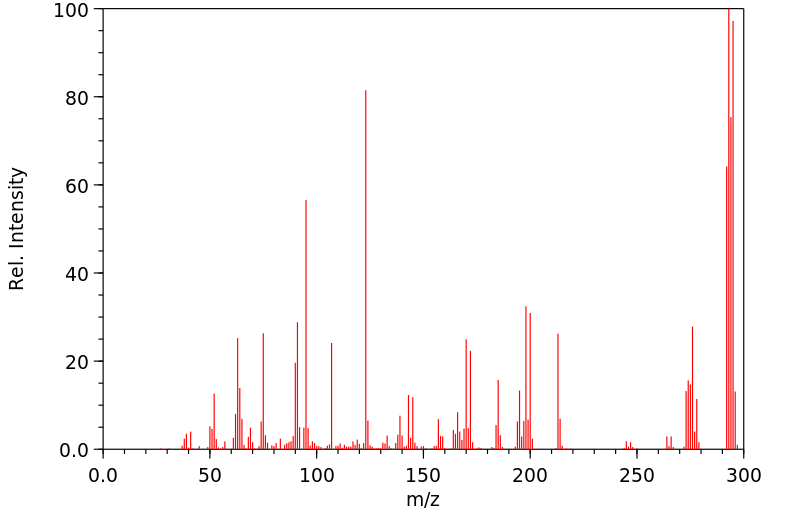
质谱MS
-
碳谱13CNMR
-
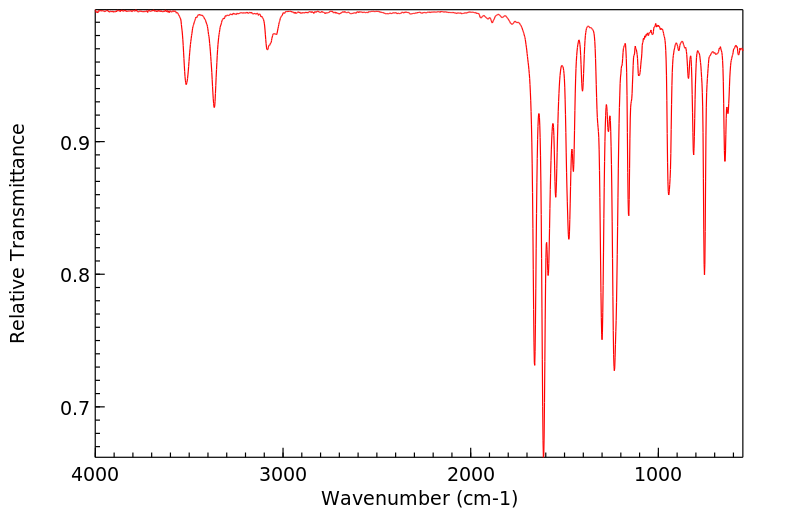
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫