2-溴-1,3-二乙基苯 | 64919-15-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:187 °C / 10mmHg
-
密度:0.96
-
保留指数:1801;1792;1793;1797;1800;1808
计算性质
-
辛醇/水分配系数(LogP):5.9
-
重原子数:17
-
可旋转键数:13
-
环数:0.0
-
sp3杂化的碳原子比例:0.928
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915900090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温
SDS
Section I.Chemical Product and Company Identification
Chemical Name 2-Chloroethyl Laurate
Portland OR
Synonym Dodecanoic acid, 2-chloroethyl ester (CA INDEX
NAME);
Lauric Acid 2-Chloroethyl Ester
Chemical Formula C14H27ClO
CAS Number 64919-15-9
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
2-Chloroethyl Laurate 64919-15-9 Min. 95.0 (GC) Not available. Not available.
Section III. Hazards Identification
No specific information is available in our data base regarding the toxic effects of this material for humans. However,
Acute Health Effects
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
Inhalation If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic
material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Not available.
Flammability May be combustible at high temperature. Auto-Ignition
Flash Points Not available. Flammable Limits Not available.
Combustion Products These products are toxic carbon oxides (CO, CO2), halogenated compounds.
WARNING: Highly toxic HCl gas is produced during combustion.
Fire Hazards
Not available.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
2-Chloroethyl Laurate
Section VI. Accidental Release Measures
Spill Cleanup Absorb with an inert material and put the spilled material in an appropriate waste disposal. Finish cleaning the spill by rinsing
any contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
Instructions
disposal.
Section VII. Handling and Storage
Handling and Storage Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the container and store in a dry, cool
place. Avoid excessive heat and light. Do not breathe gas/fumes/ vapor/spray.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their respective
Engineering Controls
threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location.
Splash goggles. Lab coat. Vapor respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a
Personal Protection
specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Liquid. (Clear, colorless.) Solubility
Physical state @ 20°C Not available.
0.96 (water=1)
Specific Gravity
262.82
Molecular Weight Partition Coefficient Not available.
Boiling Point 187°C (368.6°F) @ 10 mmHg Vapor Pressure Not available.
Not available. Not available.
Melting Point Vapor Density
1.45 Volatility Not available.
Refractive Index
Not available.
Critical Temperature Not available. Odor
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
Not available.
RTECS Number
Routes of Exposure Eye Contact. Ingestion. Inhalation.
Not available.
Toxicity Data
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
No specific information is available in our data base regarding the toxic effects of this material for humans. However,
Acute Toxic Effects
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling this
compound.
Section XII. Ecological Information
Not available.
Ecotoxicity
Environmental Fate Not available.
Continued on Next Page
2-Chloroethyl Laurate
Section XIII. Disposal Considerations
Waste Disposal Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
Not a DOT controlled material (United States).
DOT Classification
PIN Number Not applicable.
Proper Shipping Name Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not controlled under WHMIS (Canada).
(Canada)
EINECS Number (EEC) Not available.
EEC Risk Statements Not available.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,2,2-三氯月桂酸乙酯 2,2,2-Trichloroethyl dodecanoate 71071-51-7 C14H25Cl3O2 331.71 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-硫氰酸基乙基月桂酸酯 1-lauroyloxy-2-thiocyanato-ethane 301-11-1 C15H27NO2S 285.451
反应信息
-
作为反应物:描述:参考文献:名称:Selenium- and Tellurium-Catalyzed Deprotection of 2-Haloethyl Esters by Sodium Borohydride摘要:2-溴乙基和2-氯乙基酯1在温和条件下,使用少量催化的硒或碲与氢化钠反应,能够容易地去保护羧酸2,并获得良好的产率。与其他使用化学计量量的硒或碲的方法相比,该方法具有操作简单和毒性低的优势。DOI:10.1055/s-1990-26965
-
作为产物:描述:参考文献:名称:Copper(I)-Promoted Dechlorinative Surzur−Tanner Rearrangement of 2,2,2-Trichloroethyl Carboxylates摘要:[GRAPHICS]2,2,2-Trichloroethyl carboxylates undergo highly efficient dechlorinative Surzur-Tanner rearrangement with 2 equiv of a 1:1 molar mixture of CuCl and bpy in boiling DCE to give 1-chloroethenyl carboxylates in which copper appears to play an important role, probably by coordinating the initial radical or as a Lewis acid catalyst.DOI:10.1021/ol027139u
文献信息
-
Thiocyano ester申请人:ROHM &公开号:US02220521A1公开(公告)日:1940-11-05
537,815. Esters ; insecticides. ROHM &; HAAS CO. Oct. 2, 1939, No. 27025. Convention date, Nov. 8, 1938. [Class 2 (iii)] [Also in Group VI] Esters of unsubstituted glycols or polyalkylene glycols, in which one hydroxyl group is esterified with a monocarboxylic acid containing at least four carbon atoms and the other is replaced by a thiocyano radicle, are made by heating the monocarboxylic acid ester of a halohydrin with a thiocyanate. The monocarboxylic ester of the halohydrin may be prepared by esterifying the glycol (1 mol.) with the acid (1 mol.) and treating the product with phosphorus trichloride, by reacting a halide of the acid with an alkylene oxide or halohydrin, by reacting the sodium salt of the acid with an alkylene dihalide, or by esterifying the acid with a halohydrin. The replacement of the halogen atom by a thiocyano group may be effected by heating with excess of an anhydrous inorganic thiocyanate in the presence or absence of an anhydrous solvent, e.g. methyl isobutyl ketone, copper or sodium .iodide being used as a catalyst if desired. The monocarboxylic acids may be aliphatic, aromatic, arylaliphatic, cycloaliphatic or heterocyclic, the following being specified : butyric, isobutyric, crotonic, α-ethylbutyric, capric, caprylic, lauric, α-ethyl-hexoic, myristic, naphthenic, benzoic, benzyloxybenzoic, salicylic, clupanodonic, chlorobenzoic. phenylacetic, abietic, campholic, naphthylacetic, tetrahydronaphthylacetic, fluorobenzoic, oleic, linoleic, eloidic, ricinoleic, octyloxyacetic, caprylphenoxyacetic, cyclohexyloxyacetic, m-nitrobenzoic, benzoylbenzoic, acetoacetic, undecylenic, stearic, eleostearic, palmitic, and commercial fatty acid mixtures, e.g. coco-nut oil fatty acids, mixtures obtained by oxidation of petroleum, and tall oils. As glycols, ethylene, propylene and butylene glycols, 1 : 10-decanediol, and diethylene ; triethylene and dibutylene glycols are specified. In examples products are obtained from (1) ethylene chlorhydrin, sodium thiocyanate and (a) furoic acid, (b) benzoic acid, (c) lauric acid, (d) technical coco-nut oil acid, (e) a commercial mixture of fatty acids obtained by oxidation of petroleum, and (f) naphthenic acid, (2) sodium isobutyrate is heated with #: #<;SP>;1<;/SP>;-dichlorodiethyl ether, and the product heated with sodium thiocyanate, and (3) the sodium salt of coco-nut oil acid is heated with #: #<;SP>;1<;/SP>;-dichlorodiethyl ether, and the product heated with sodium thiocyanate. The products are useful as insecticides ; they may be dissolved in kerosene to produce insect sprays, or mixed with talc to produce insecti- .cidal dusting powders. They may also be dissolved in a light oil and emulsified in water to produce agricultural sprays. They may be used in admixture with other toxic materials, such as other organic thiocyanates, rotenone, derris extract, pyrethrum, nitro-substituted phenylbenzyl ethers, or nitro-substituted diphenyl ethers. Specification 361,900 is referred to.
537,815. 酯类;杀虫剂。ROHM & HAAS CO. 1939年10月2日,编号27025。公约日期,1938年11月8日。[2类(iii)] [也在第六组中] 未取代的乙二醇或聚烷基乙二醇的酯类,其中一个羟基与至少含有四个碳原子的一元羧酸酯化,另一个被硫氰基取代,通过将一元羧酸酯与硫氰酸盐加热制备。一元羧酸酯可以通过将乙二醇(1摩尔)与酸(1摩尔)酯化并用三氯化磷处理产物,通过将酸的卤化物与烷氧化物或卤水合物反应,通过将酸的钠盐与烷二卤化物反应,或通过将酸与卤水合物酯化来制备。将卤素原子替换为硫氰基可以通过在无水无机硫氰酸盐的过量存在下加热,在无水溶剂的存在或缺席下进行,例如甲基异丁基酮,如果需要,可使用铜或钠碘化物作为催化剂。一元羧酸可以是脂肪的、芳香的、芳基脂肪的、环脂肪的或杂环的,具体如下:丁酸、异丁酸、巴豆酸、α-乙基丁酸、癸酸、辛酸、月桂酸、α-乙基己酸、肉豆蔻酸、环烷酸、苯甲酸、苄氧基苯甲酸、水杨酸、鱼腥草酸、氯苯甲酸、苯乙酸、松香酸、萜酸、萘乙酸、四氢萘乙酸、氟苯甲酸、油酸、亚油酸、油酸、蓖麻油酸、辛氧基乙酸、辛基苯氧乙酸、环己氧基乙酸、m-硝基苯甲酸、苯甲酰苯甲酸、乙酰乙酸、十一烯酸、硬脂酸、亚油酸、棕榈酸和商业脂肪酸混合物,例如椰子油脂肪酸、由石油氧化得到的混合物和松香油。作为乙二醇,指定了乙烯、丙烯和丁烯乙二醇、1:10-癸二醇和二乙二醇;三乙二醇和二丁烯乙二醇。在示例中,从(1)乙烯氯水合物、硫氰酸钠和(a)呋酸、(b)苯甲酸、(c)月桂酸、(d)技术椰子油酸、(e)由石油氧化得到的商业脂肪酸混合物和(f)环烷酸中获得产品,(2)异丁酸钠与#:#<;SP>;1<;/SP>;-二氯二乙醚加热,产物与硫氰酸钠加热,以及(3)椰子油酸钠盐与#:#<;SP>;1<;/SP>;-二氯二乙醚加热,产物与硫氰酸钠加热。产品可用作杀虫剂;它们可以溶解在煤油中制成杀虫喷雾,或与滑石混合以制成杀虫粉末。它们也可以溶解在轻质油中,并乳化在水中制成农业喷雾。它们可以与其他有毒材料混合使用,例如其他有机硫氰酸盐、罗汤宁、德里斯提取物、除虫菊、硝基取代的苯基苯基醚或硝基取代的二苯基醚。参考规范361,900。 -
Sustained release pharmaceutical compositions申请人:Zhang Yongfeng Jack公开号:US20070154546A1公开(公告)日:2007-07-05The present invention relates to a pharmaceutical composition comprising a salt of quaternary ammonium of an acid drug in the form of a suspension or an emulsion, suitable for parenteral administration and providing a sustained release of the drug. In one preferred embodiment, the present invention is directed to a long term sustained release composition for parenteral administration, comprising a salt of heparin or low molecular weight heparin in an emulsion with a salt of acylcholine, for the prevention and treatment of venous thrombosis and pulmonary embolism.
-
Zinc-Catalyzed Depolymerization of Polyethers to Produce Valuable Building Blocks作者:Stephan EnthalerDOI:10.1007/s10562-014-1214-8日期:2014.5a straightforward methodology for the depolymerization of artificial polyethers applying cheap and abundant zinc(II) salts as precatalysts. In the presence of bio-based fatty acid chlorides as depolymerization reagent well-defined chloroesters were accessible in good to excellent yields. Moreover, acetic anhydride and fatty acids were applied as depolymerization reagents resulting in the formation of
-
Cationic Imidazolium Monomeric Surfactants: Their Synthesis and Surface Active Properties作者:Ishtiaque Ahmad、Pankaj Patial、Charanjeet Kaur、Satindar KaurDOI:10.1007/s11743-013-1527-4日期:2014.3alcohols. The surface activity of the molecules has been determined by measurement of their conductance and surface tension in aqueous solution. The dynamics of surface activity of these surfactants have also been investigated in the presence of sodium halides (NaCl and NaBr) by surface tension measurement. A series of useful parameters like critical micelle concentration (CMC), surface tension at the使用脂肪酸和卤代醇等可再生原料合成了一系列长链酯基水溶性阳离子。分子的表面活性已经通过测量其在水溶液中的电导率和表面张力来确定。还通过表面张力测量在卤化钠(NaCl和NaBr)存在下研究了这些表面活性剂的表面活性动力学。一系列象临界胶束浓度(CMC),在CMC(γ表面张力有用的参数CMC),吸附效率(PC 20),表面张力降低的有效性(Π CMC),所述胶束(Δ的吉布斯自由能ģ 0mic)和吉布斯吸附自由能(ΔG 0ads)已通过表面张力和电导率方法获得的测量值确定。进一步与吉布斯吸附等温线的应用中,最大表面过量浓度(Γ最大)和最小表面积/分子(A分钟在空气-水界面)也估计。这些长链阳离子的热稳定性已通过在氮气氛下的热重分析法进行了测量。热稳定性测量的分析表明,这些长链咪唑的热稳定性随着链长的增加而增加。
-
[EN] NEURAMINIDASE INHIBITOR COMPOUND, AND PHARMACEUTICAL COMPOSITION AND USE THEREOF<br/>[FR] COMPOSÉ INHIBITEUR DE LA NEURAMINIDASE, COMPOSITION PHARMACEUTIQUE ET UTILISATION DE CELUI-CI<br/>[ZH] 神经氨酸酶抑制剂类化合物、其药物组合物及其用途
表征谱图
-
氢谱1HNMR
-
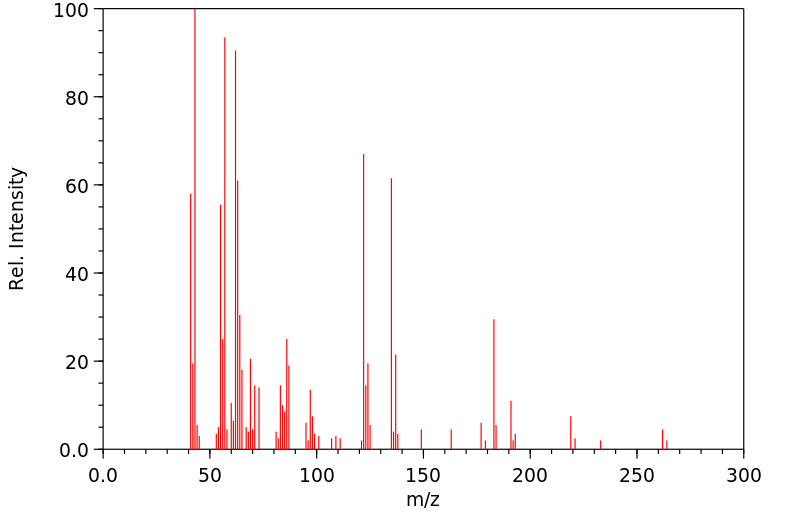
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息