2-溴庚酸乙酯 | 5333-88-0
中文名称
2-溴庚酸乙酯
中文别名
——
英文名称
ethyl 2-bromoheptanoate
英文别名
2-bromo-n-heptanoic acid ethyl ester;ethyl α-bromo heptanoate;(+/-)-ethyl 2-bromoheptanoate;ethyl-2-bromoheptanoate;2-bromo-heptanoic acid ethyl ester;2-Brom-heptansaeure-aethylester
CAS
5333-88-0
化学式
C9H17BrO2
mdl
——
分子量
237.137
InChiKey
GNCLPIAYAPQPOU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:109 °C10 mm Hg(lit.)
-
密度:1.211 g/mL at 25 °C(lit.)
-
闪点:220 °F
-
保留指数:1282;1382
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:12
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.89
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:8
-
安全说明:S26,S27,S28,S36/37/39,S45
-
危险类别码:R34,R36/37
-
海关编码:2915900090
-
包装等级:III
-
危险类别:8
-
危险性防范说明:P261,P264,P271,P280,P302+P352,P304+P340+P312,P305+P351+P338,P310,P332+P313,P362,P403+P233,P405,P501
-
危险品运输编号:3265
-
危险性描述:H315,H318,H335
-
储存条件:存于室温、避光且在惰性气体保护下
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl 2,2-dibromoheptanoate 380500-55-0 C9H16Br2O2 316.033
反应信息
-
作为反应物:描述:2-溴庚酸乙酯 在 lithium diisopropyl amide 、 1,1,2,2-四氟-1,2-二溴乙烷 作用下, 以 四氢呋喃 、 正己烷 为溶剂, 反应 1.0h, 以86%的产率得到ethyl 2,2-dibromoheptanoate参考文献:名称:Synthesis of .ALPHA.,.ALPHA.-Dibromo Esters as Precursors of Ynolates.摘要:脂肪族α,α-二溴酯作为ynolate的前体,通过将锂α-溴酯enolate与1,2-二溴四氟乙烷进行溴化反应,以良好的产率合成。α-三甲基硅基-α,α-二溴酯则通过自由基溴化法合成。DOI:10.1248/cpb.51.477
-
作为产物:参考文献:名称:Sun, Zhaoyun; Peng, Xinhua; Dong, Xiongzi, Asian Journal of Chemistry, 2012, vol. 24, # 2, p. 929 - 930摘要:DOI:
文献信息
-
An efficient synthesis of (E)-α,β-unsaturated ketones and esters with total stereoselectivity by using chromium dichloride作者:José M. Concellón、Humberto Rodríguez-Solla、Carmen MéjicaDOI:10.1016/j.tet.2006.01.052日期:2006.4(E)-α,β-Unsaturated ketones 1 or esters 2 can be obtained with complete stereoselectivity by reaction of different 2-chloro-3-hydroxy ketones 3 or esters 4 and CrCl2. A comparative study of the results of synthesis of ketones 1 with CrCl2 or samarium is performed. A mechanism to explain both β-elimination reactions has been proposed.(E)-α,β-不饱和酮1或酯2可以通过不同的2-氯-3-羟基酮3或酯4和CrCl 2的反应以完全的立体选择性获得。对CrCl 2或sa合成酮1的结果进行了比较研究。已经提出了解释两种β-消除反应的机制。
-
N-hydroxy-2-(Alkyl,Aryl or Heteroaryl sulfanyl, sulfinyl or sulfonyl) 3-substituted alkyl, aryl or heteroarylamides as matrix metalloproteinase inhibitors申请人:American Cyanamid Company公开号:US06342508B1公开(公告)日:2002-01-29Matrix metalloproteinases (MMps) are a group of enzymes that have been implicated in the pathological destruction of connective tissue and basement membranes. These zinc containing endopeptidases consist of several subsets of enzymes including collagenases, stromelysins and gelatinases. TNF-&agr; converting enzyme (TACE), a pro-inflammatory cytokine, catalyzes the formation of TNF-&agr; from membrane bound TNF-&agr; precursor protein. It is expected that small molecule inhibitors of MMPs and TACE therefore have the potential for treating a variety of disease states. The present invention provides low molecular weight, non-peptide inhibitors of matrix metalloproteinases (MMPs) and TNF-&agr; converting enzyme (TACE) for the treatment of arthritis, tumor metastasis, tissue ulceration, abnormal wound healing, periodontal disease, bone disease, diabetes (insulin resistance) and HIV infection. The compounds of this invention are represented by the formula where R1, R2, R3 and R4 are described herein.翻译结果如下: 基质金属蛋白酶(MMPhref=https://www.molaid.com/MS_61907 target="_blank">MMps)是一组与连接组织和基底膜病理破坏有关的酶。这些含有锌的内切肽酶包括几个酶亚组,如胶原酶、溶素和明胶酶。肿瘤坏死因子-α转化酶(TACE),一种促炎症细胞因子,催化膜结合的肿瘤坏死因子-α前体蛋白形成肿瘤坏死因子-α。因此,人们预期基质金属蛋白酶(MMPs)和TACE的小分子抑制剂可能具有治疗多种疾病状态的前景。本发明提供了低分子量、非肽类的基质金属蛋白酶(MMPs)和肿瘤坏死因子-α转化酶(TACE)的抑制剂,用于治疗关节炎、肿瘤转移、组织溃疡、异常伤口愈合、牙周病、骨病、糖尿病(胰岛素抵抗)和HIV感染。本发明中的化合物由以下公式表示: 其中R1、R2、R3和R4在本说明书中有所描述。
-
Oxidation of Diols and Ethers by NaBrO<sub>3</sub>/NaHSO<sub>3</sub>Reagent作者:Satoshi Sakaguchi、Daisuke Kikuchi、Yasutaka IshiiDOI:10.1246/bcsj.70.2561日期:1997.10NaBrO3 combined with NaHSO3 was found to be an excellent oxidizing reagent of alcohols, diols, and ethers under mild conditions. A variety of aliphatic and cyclic diols were selectively oxidized with satisfactory yields to the corresponding hydroxy ketones and/or diketones, which are difficult to selectively prepare due to a concomitant formation of cleaved products. For example, 2-hydroxycyclohexanone and 1,2-cyclohexanedione were selectively formed by allowing 1,2-cyclohexanediol to react with NaBrO3/NaHSO3 reagent in a selected solvent. On the other hand, an alkyl ether, such as dioctyl ether, reacted with NaBrO3/NaHSO3 in water at room temperature to give octyl octanoate in 82% yield. The same oxidation at higher temperature (60 °C) produced the α-brominated ester, octyl 2-bromooctanoate, which is considered to be formed through an alkenyl alkyl ether as the intermediate. The treatment of 1-ethoxy-1-heptene with NaBrO3/NaHSO3 afforded ethyl 2-bromoheptanoate and 2-bromoheptanoic acid as the major products.NaBrO3与NaHSO3组合被发现是一种在温和条件下对醇、二醇和醚类具有优异氧化能力的试剂。多种脂肪族和环状二醇被选择性地氧化为相应的羟基酮和/或二酮,产率令人满意,这些产物由于伴随生成断裂产物而难以选择性制备。例如,通过在选定溶剂中使1,2-环己二醇与NaBrO3/NaHSO3试剂反应,选择性地生成了2-羟基环己酮和1,2-环己二酮。另一方面,在室温下,二辛醚在水中的NaBrO3/NaHSO3反应以82%的产率得到了辛基辛酸酯。同样的氧化反应在较高温度(60°C)下产生了α-溴代酯,即辛基2-溴辛酸酯,这被认为是通过烯基烷基醚作为中间体形成的。使用NaBrO3/NaHSO3处理1-乙氧基-1-庚烯,主要产物是乙基2-溴庚酸酯和2-溴庚酸。
-
Synthesis and characterization of novel phosphonocarboxylate inhibitors of RGGT作者:Fraser P. Coxon、Łukasz Joachimiak、Arafath Kaja Najumudeen、George Breen、Joanna Gmach、Christina Oetken-Lindholm、Rebecca Way、James E. Dunford、Daniel Abankwa、Katarzyna M. BłażewskaDOI:10.1016/j.ejmech.2014.06.062日期:2014.9selective inhibitors of Rab geranylgeranyl transferase (RabGGTase, RGGT), an enzyme implicated in several diseases including ovarian, breast and skin cancer. Here we present the synthesis and biological characterization of an extended set of this class of compounds, including lipophilic derivatives of the known RGGT inhibitors. From this new panel of PCs, we have identified an inhibitor of RGGT that is
-
Etude de la reaction chlorocarbene-acetals de cetenes作者:N. Slougui、G. RousseauDOI:10.1016/s0040-4020(01)96366-5日期:1985.1The reaction of chloro, chloromethyl and chlorophenyl carbenoids with ketene alkylsilylacetals has been studied. Excellent yields of cyclopropanation were observed and the unstable chlorocyclopropanone acetals formed were thermally rearranged in high yield into α-substituted α,β-ethylenic esters. This new method for the synthesis of unsaturated esters appeared complementary of the known-ones.
表征谱图
-
氢谱1HNMR
-
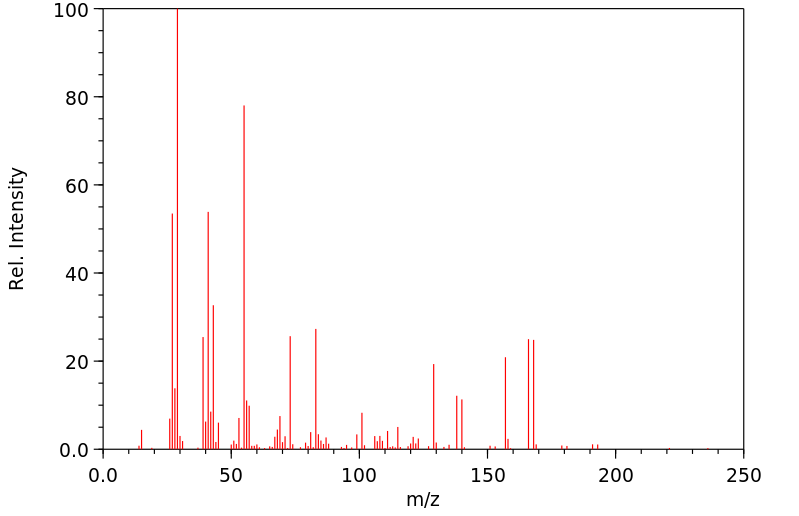
质谱MS
-
碳谱13CNMR
-
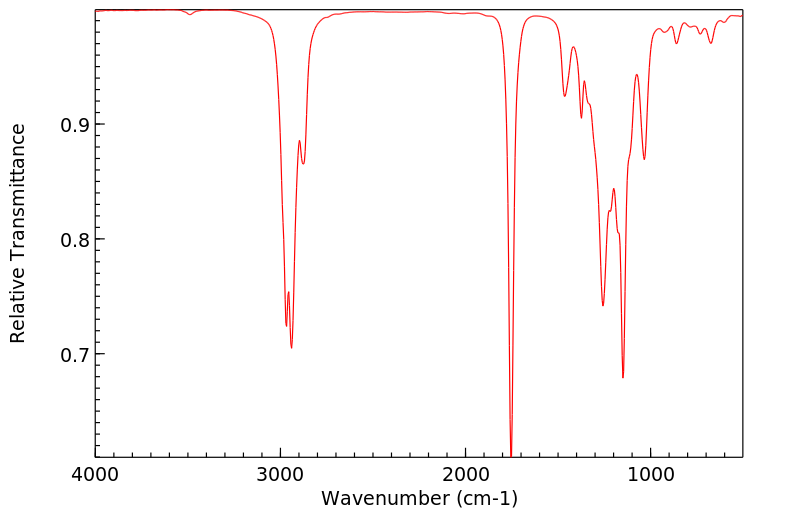
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯