2-甲基-3-氨基苯甲酸 | 52130-17-3
中文名称
2-甲基-3-氨基苯甲酸
中文别名
3-氨基邻甲苯酸;3-氨基-2-甲基苯甲酸;3-氨基邻甲苯甲酸;3-氨基邻甲苯腈
英文名称
3-amino-2-methylbenzoic acid
英文别名
2-methyl-3-aminobenzoic acid
CAS
52130-17-3
化学式
C8H9NO2
mdl
MFCD00075026
分子量
151.165
InChiKey
BYHMLZGICSEKIY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:178-181 °C(lit.)
-
沸点:273.17°C (rough estimate)
-
密度:1.2023 (rough estimate)
-
溶解度:在热甲醇中几乎透明
-
稳定性/保质期:
如果按照规定使用和储存,则不会分解,也未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:63.3
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2922499990
-
危险品运输编号:NONH for all modes of transport
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:请将贮藏器密封保存,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
| Name: | 3-Amino-2-Methylbenzoic Acid 97% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 52130-17-3 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 52130-17-3 | 3-Amino-2-Methylbenzoic Acid | 97% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 52130-17-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 178 - 181 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H9NO2
Molecular Weight: 151.16
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 52130-17-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Amino-2-Methylbenzoic Acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 52130-17-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 52130-17-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 52130-17-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质:白色粉末,熔点为178-181℃。
用途:用作医药中间体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氨基-2-甲基苯甲酸甲酯 methyl 3-amino-2-methylbenzoate 18583-89-6 C9H11NO2 165.192 2-甲基-3-硝基苯甲酸 2-methyl-3-nitrobenzic acid 1975-50-4 C8H7NO4 181.148 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-氨基-2-甲基苯甲酸甲酯 methyl 3-amino-2-methylbenzoate 18583-89-6 C9H11NO2 165.192 3-氨基-2-甲基苯甲醇 3-amino-2-methylbenzyl alcohol 83647-42-1 C8H11NO 137.181 —— 3-N-(Methylsulfonyl)amino-2-methyl benzoic acid 168899-41-0 C9H11NO4S 229.257 —— 2-Methyl-3-[(trifluoroacetyl)amino]benzoic acid 915094-44-9 C10H8F3NO3 247.174 —— methyl 3-(cyclopentylamino)-2-methylbenzoate 1157319-96-4 C14H19NO2 233.31 —— 3-[Cyclopentyl(methyl)amino]-2-methylbenzoic acid 1403595-61-8 C14H19NO2 233.31
反应信息
-
作为反应物:描述:2-甲基-3-氨基苯甲酸 在 四(三苯基膦)钯 盐酸 、 sodium tetrahydroborate 、 氯化亚砜 、 硫酸 、 sodium hydride 、 potassium carbonate 、 三乙胺 、 sodium nitrite 作用下, 以 四氢呋喃 、 甲醇 、 二氯甲烷 、 水 、 溶剂黄146 、 异丙醇 、 乙腈 为溶剂, -60.0~50.0 ℃ 、344.75 kPa 条件下, 反应 43.5h, 生成 Tert-butyl 2-(3-(methoxycarbonyl)-2-methylphenyl)pyrrolidine-1-carboxylate参考文献:名称:WO2006/117669摘要:公开号:
-
作为产物:描述:参考文献:名称:Viscoelasticity, dielectric anisotropy, and birefringence in the nematic phase of three four-ring bent-core liquid crystals with an L-shaped molecular frame摘要:分子形状是决定热致液晶(LC)材料性质的重要因素。我们合成并研究了几种由不对称弯曲分子构成的液晶化合物,这些分子具有一个形状像字母“L”的刚性四环核心。我们测量了介电常数、双折射、撓曲 K1 和弯曲 K3 弹性常数、撕裂粘度 ηsplay 以及流动粘度 η|| 和 η⊥ 的温度依赖性。弯曲-撕裂各向异性 δK31 = K3 − K1 为负,类似于由对称弯曲的 V 形分子形成的向列型液晶的情况。介电各向异性 Δε 和双折射在整个向列相范围内均为正。撕裂粘度 ηsplay 和流动粘度 η|| 及 η⊥ 小于在类似温度下测得的对称 V 形弯曲核心材料的粘度。比率 Γ = ηsplay/η||,⊥ 在 5-4 范围内,这是典型的杆状液晶的特征。报道的 L 形弯曲核心向列型液晶结合了弯曲核心液晶的有用特征(例如负的 δK31,适合于广范围蓝相的形成)与相对较低的粘度,这是杆状液晶的典型特性,对于电光开关应用具有益处。DOI:10.1039/c2sm26448j
文献信息
-
[EN] VIRAL POLYMERASE INHIBITORS<br/>[FR] INHIBITEURS DE POLYMERASE VIRALE申请人:BOEHRINGER INGELHEIM INT公开号:WO2004065367A1公开(公告)日:2004-08-05An isomer, enantiomer, diastereoisomer or tautomer of a compound, represented by formula (I): wherein wherein A, B, R2, R3, L, M1, M2, M3, M4, Y1, Y0, Z and Sp are as defined in claim 1, or a salt thereof, as an inhibitor of HCV NS5B polymerase.
-
[EN] METABOLICALLY ROBUST ANALOGS OF CYP-EICOSANOIDS FOR THE TREATMENT OF CARDIAC DISEASE<br/>[FR] ANALOGUES ROBUSTES SUR LE PLAN MÉTABOLIQUE DES CYP-EICOSANOÏDES POUR LE TRAITEMENT DES MALADIES CARDIAQUES申请人:OMEICOS THERAPEUTICS GMBH公开号:WO2017013265A1公开(公告)日:2017-01-26The present invention relates to compounds according to general formula (I) which are metabolically robust analogues of bioactive lipid mediators derived from omega-3 polyunsaturated fatty acids (n-3 PUFAs).The present invention further relates to compositions containing one or more of these compounds and to the use of these compounds or compositions for the treatment or prevention of cardiovascular diseases.
-
Insecticidal N'-substituted-N,N'-diacylhydrazines申请人:Rohm and Haas Company公开号:US05530028A1公开(公告)日:1996-06-25Insecticidal compounds having the formula N-(2-R.sup.a -3-R.sup.b -4-R.sup.h -benzoyl)-N'-(2-R.sup.c -3-R.sup.d -4-R.sup.e -5-R.sup.f -benzoyl)-N'-R.sup.g -hydrazine wherein R.sup.a is a halo or lower alkyl; R.sup. b is lower alkoxy, optionally substituted with halo (preferably fluoro); R.sup.c is selected from hydrogen, halo, lower alkyl, lower alkoxy, lower alkoxy lower alkyl, and nitro; R.sup.d, R.sup.e and R.sup.f are each independently selected from hydrogen, bromo, chloro, fluoro, lower alkyl, lower alkoxy, and lower alkoxy lower alkyl; R.sub.g is a (C.sub.4 -C.sub.6)alkyl; R.sup.h is hydrogen, lower alkoxy, lower alkyl, or when taken together with R.sup.b is methylenedioxy (--OCH.sub.2 O--), 1,2-ethylenedioxy (--OCH.sub.2 CH.sub.2 O--), 1,2-ethyleneoxy (--CH.sub.2 CH.sub.2 O--) or 1,3-propyleneoxy (--CH.sub.2 CH.sub.2 CH.sub.2 O--) wherein an oxo atom is located at the R.sup.b position; and the substituents R.sup.c and R.sup.d, or R.sup.d and R.sup.e, or R.sup.e and R.sup.f when taken together can be methylenedioxy or 1,2-ethylenedioxy as well as compositions comprising an agronomically acceptable carrier and an insecticidally effective amount of such compounds; and methods of using such compounds and compositions. Also, methods for the production of the compounds and their intermediates, which methods comprise either admixing a 3-amino-2-(substituted)-benzoic acid, sodium nitrite and methanol under acidic conditions or admixing a 3,4-fused heterocyclic benzoic acid and an alkyl lithium reagent followed by subsequent reaction with an electrophilic reagent.具有以下结构的杀虫化合物:N-(2-R.sup.a -3-R.sup.b -4-R.sup.h -苯甲酰基)-N'-(2-R.sup.c -3-R.sup.d -4-R.sup.e -5-R.sup.f -苯甲酰基)-N'-R.sup.g -肼,其中R.sup.a是卤素或较低的烷基;R.sup.b是较低的烷氧基,可选择性地取代为卤素(最好是氟);R.sup.c从氢、卤素、较低的烷基、较低的烷氧基、较低的烷氧基较低的烷基和硝基中选择;R.sup.d、R.sup.e和R.sup.f分别独立地从氢、溴、氯、氟、较低的烷基、较低的烷氧基和较低的烷氧基较低的烷基中选择;R.sub.g是(C.sub.4 -C.sub.6)烷基;R.sup.h是氢、较低的烷氧基、较低的烷基,或者与R.sup.b一起取时是亚甲二氧基(--OCH.sub.2 O--)、1,2-乙二氧基(--OCH.sub.2 CH.sub.2 O--)、1,2-乙烯氧基(--CH.sub.2 CH.sub.2 O--)或1,3-丙烯氧基(--CH.sub.2 CH.sub.2 CH.sub.2 O--),其中氧原子位于R.sup.b位置;取代基R.sup.c和R.sup.d,或R.sup.d和R.sup.e,或R.sup.e和R.sup.f在一起可以是亚甲二氧基或1,2-乙二氧基,以及包含农学上可接受的载体和这类化合物的杀虫有效量的组合物;以及使用这类化合物和组合物的方法。此外,还提供了制备这些化合物及其中间体的方法,这些方法包括在酸性条件下混合3-氨基-2-(取代)-苯甲酸、亚硝酸钠和甲醇,或者混合3,4-融合杂环苯甲酸和烷基锂试剂,然后与亲电试剂进行后续反应。
-
Derivatives of purine, their preparation process and pharmaceutical compositions containing them申请人:Haesslein Jean-Luc公开号:US20050187228A1公开(公告)日:2005-08-25A compound of the formula wherein R is defined as in the specification, which compounds have an inhibitory effect vis-à-vis cycline-dependent kinase proteins (cdk) and are endowed with antimitotic properties.其中R在规范中定义,该化合物具有对细胞周期依赖性激酶蛋白(cdk)具有抑制作用并具有抗有丝分裂特性。
-
[EN] ALKYNYL ARYL CARBOXAMIDES<br/>[FR] ALKYNYLARYL-CARBOXAMIDES申请人:APPLIED RESEARCH SYSTEMS公开号:WO2005012280A1公开(公告)日:2005-02-10The present invention is related to alkynyl aryl carboxamides of Formula (I’) and use thereof for the treatment and/or prevention of an inflammatory disorder, obesity and/or metabolic disorders mediated by insulin resistance or hyperglycemia, comprising diabetes type I and/or II, inadequate glucose tolerance. insulin resistance, hyperlipidemia, hypertriglyceridemia- hypercholesterolemia, polycystic ovary syndrome (PCOS). In particular, the present invention is related to the use of alkynyl aryl carboxamides of Formula (I’) to modulate, notably to inhibit the activity of PTPs. (I’) A is a C2-C15 alkynyl, C2-C6-alkynyl aryl, C2-C6-alkynyl heteroaryl. Cy is an aryl, heteroaryl, cycloalkyl or heterocycle group; n is either 0 or 1. Cy' is an aryl., which may optionally be fused by a 3-8 membered cycloalkyl. R1and R2 are independently from each other is selected from the group consisting of hydrogen or (CI-C6)alkyl. R4and R5 are each independently from each other selected from the group consisting of H, hydroxy. C1 -C6 alkyl, carboxy, C1-C6 alkoxy, C1 -C3 alkyl carboxy, C2-C3 alkenyl carboxy, C2-C3 alkynyl carboxy, amino or R4 and R5 may form an unsaturated or saturated heterocyclic ring, whereby at least one of R4 or R5 is not a hydrogen or C1-C6 alkyl.本发明涉及式(I')的炔基芳基羧酰胺及其用于治疗和/或预防由胰岛素抵抗或高血糖介导的炎症疾病、肥胖和/或代谢紊乱的使用,包括I型和/或II型糖尿病、葡萄糖耐量不足、胰岛素抵抗、高脂血症、高甘油三酯血症-高胆固醇血症、多囊卵巢综合征(PCOS)。特别是,本发明涉及使用式(I')的炔基芳基羧酰胺来调节,尤其是抑制PTPs的活性。(I') A是C2-C15炔基,C2-C6-炔基芳基,C2-C6-炔基杂芳基。Cy是芳基、杂芳基、环烷基或杂环族;n是0或1。Cy'是一个芳基,它可以选择性地通过一个3-8成员的环烷基融合。R1和R2独立地互选自由氢或(C1-C6)烷基组成的组。R4和R5各自独立地互选自H、羟基。C1-C6烷基、羧基、C1-C6烷氧基、C1-C3烷基羧基、C2-C3烯基羧基、C2-C3炔基羧基、氨基或R4和R5可以形成一个不饱和或饱和的杂环,其中至少R4或R5不是氢或C1-C6烷基。
表征谱图
-
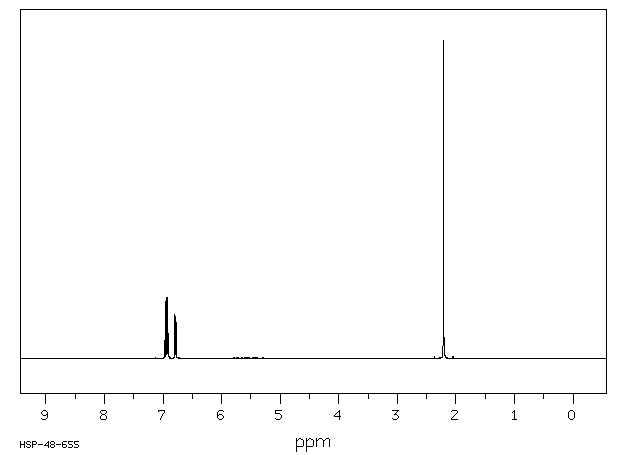
氢谱1HNMR
-
质谱MS
-
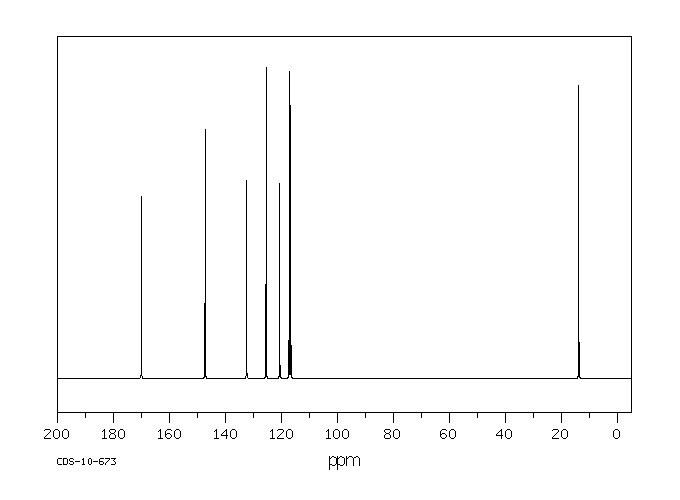
碳谱13CNMR
-
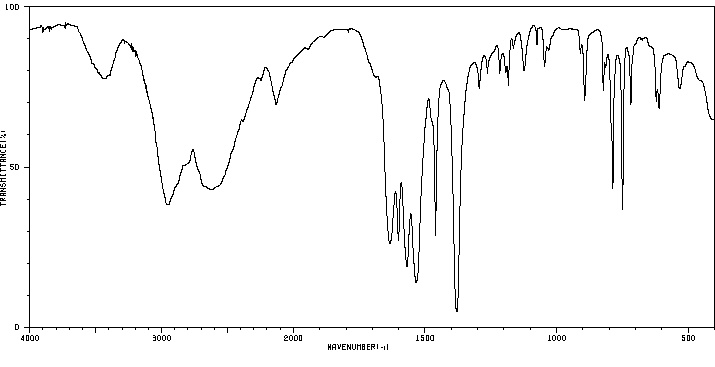
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫