2-甲氧基-5-甲基-1,4-苯醌 | 614-13-1
中文名称
2-甲氧基-5-甲基-1,4-苯醌
中文别名
——
英文名称
2-methoxy-5-methyl-1,4-benzoquinone
英文别名
2-methoxy-5-methylcyclohexa-2,5-diene-1,4-dione;2-methoxy-5-methylbenzoquinone
CAS
614-13-1
化学式
C8H8O3
mdl
——
分子量
152.15
InChiKey
VJVOAGLABGZRSL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:存放在室温、干燥且密封的环境中。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-羟基-5-甲基苯醌 4-hydroxy-5-methylcyclohexa-3,5-diene-1,2-dione 615-91-8 C7H6O3 138.123 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-Methoxy-5-methyl-6-farnesyl-1,4-benzochinon 20801-85-8 C23H32O3 356.505 —— 2-Methoxy-5-methyl-3-decaprenyl-1,4-benzochinon —— C58H88O3 833.335 —— 2-Methoxy-5-methyl-6-geranyl-1,4-benzochinon 20839-93-4 C18H24O3 288.387 —— 5-demethoxyubiquinone-2 20839-93-4 C18H24O3 288.387 —— 5-Methoxy-2-methyl-3-pentyl-[1,4]benzoquinone 129332-70-3 C13H18O3 222.284
反应信息
-
作为反应物:描述:2-甲氧基-5-甲基-1,4-苯醌 在 三氟化硼乙醚 sodium hydroxide 作用下, 以 1,4-二氧六环 为溶剂, 反应 22.75h, 生成 trans-2-methoxy-4a-methyl-4a,5,8,8a-tetrahydro-1,4-naphthoquinone参考文献:名称:紫杉醇C,D环系统的合成:α-甲氧基酮的光解摘要:描述了用于合成紫杉醇的C和D环的模型系统,其涉及α-甲氧基酮的光解。紫杉醇常见的顺式稠合的氧杂环丁醇与新型的反式稠合的氧杂环丁烷一起形成。还描述了有关此过程的选择性以及机理假设的实验。DOI:10.1016/s0040-4020(01)91212-8
-
作为产物:描述:参考文献:名称:可见光连续流光反应器中苯酚的有机光催化有氧氧化摘要:本文描述了通过使用可见光和加压空气的连续流动光反应器实现的温和光催化苯酚氧化。通过精确控制主要工艺参数,以高产率获得了广泛应用的产品,包括合成维生素。反应器设计允许低有机光催化剂负载以产生单线态氧。预计有效的有氧苯酚氧化成苯醌和对羟基苯酚有助于可持续合成。DOI:10.1002/chem.202101313
文献信息
-
Cationic [5+2] cycloaddition reactions promoted by trimethylsilyl triflate in highly polar media
-
A synthesis of gymnomitrol作者:George Büchi、Ping-Sun ChuDOI:10.1016/0040-4020(81)80018-x日期:1981.1Condensation of 1,2 - dimethylcyclopentene 10 with 2 - methyl - 4,4,5 - trimethoxycyclohexa - 2,5 - dienone 7 in methylene chloride - nitromethane with added stannic chloride gave a mixture of the two diastereomeric bicyclo[3.2.1]octanes 13 and 14 by ionic [4 + 2)cycloaddition. After selective reduction of the saturated carbonyl group with sodium borohydride, and hydrogenation of the double bond the
-
Oxidation of Methoxy- and/or Methyl-Substituted Benzenes and Naphthalenes to Quinones and Phenols by H<sub>2</sub>O<sub>2</sub>in HCOOH作者:Hideo Orita、Masao Shimizu、Takashi Hayakawa、Katsuomi TakehiraDOI:10.1246/bcsj.62.1652日期:1989.5The oxidation of a number of arenes (methoxybenzenes, methylbenzenes, and naphthalenes) to quinones and phenols by H2O2 in HCOOH has been examined. Methoxybenzenes were much more easily oxidized to p-benzoquinones than methylbenzenes (e.g., 1,3,5-trimethoxybenzene was oxidized to 2,6-dimethoxy-p-benzoquinone in a 75% yield and 1,2,4-trimethylbenzene to 2,3,5-trimethyl-p-benzoquinone in a 16% yield)
-
Regiodivergent oxidation of alkoxyarenes by hypervalent iodine/oxone® system作者:Hideyasu China、Kokoro Tanihara、Hirotaka Sasa、Kotaro Kikushima、Toshifumi DohiDOI:10.1016/j.cattod.2019.08.060日期:2020.5compound, i.e., 2-iodobenzoic acid (2-IB), selectively yields p-quinones from monomethoxyarenes under mild conditions. In this reaction system, an organoiodine compound is immediately oxidized by Oxone® to generate cyclic hypervalent iodine (III) species in situ, which serves as the specific mediator for the selective p-quinone synthesis, preventing o-quinone formation.
-
Anodic Oxidation of Mono- and Disubstituted 1,4-Dimethoxybenzenes作者:Cheng-Chu Zeng、James Y. BeckerDOI:10.1021/jo0302253日期:2004.2.1disubstituted ones. Controlled-potential oxidation at the glassy carbon anodes and in a neutral LiClO4−methanol medium led to more complex mixtures of products, namely, polymers and new coupling products from monosubstituted substrates and quinones and side-chain oxidation (or substitution) products from the disubstituted ones. Three new coupling products were isolated and characterized by X-ray measurements单的电化学氧化和二取代的1,4-二甲氧基苯衍生物与零个,一个,和两个苄基CH 2 X基团(X = OAC,氯,OH)(图5a - ç和图6a - c)中已通过使用进行在甲醇中以及在存在不同电解质和工作电极的情况下进行恒流和控制电位技术的研究。在KOH-甲醇溶液中恒流电解主要从5a - c和6b生成相应的1,4-醌衍生物,而双取代的1,4-二甲氧基苯6a,c进行侧链氧化形成2,5-二甲氧基对苯二甲醛。在将介质从常用的碱性KOH-甲醇更改为中性LiClO 4-甲醇后,在大多数情况下获得了新的产品范围,涉及单取代底物的新型偶联产物和双取代底物的醌衍生物。在玻璃碳阳极和中性LiClO 4-甲醇介质中的受控电势氧化导致产物的更复杂混合物,即单取代的底物和醌的聚合物和新的偶联产物,以及来自碳原子的侧链氧化(或取代)产物。双取代的。分离了三种新的偶联产物,并通过X射线测量对其进行了表征。
表征谱图
-
氢谱1HNMR
-
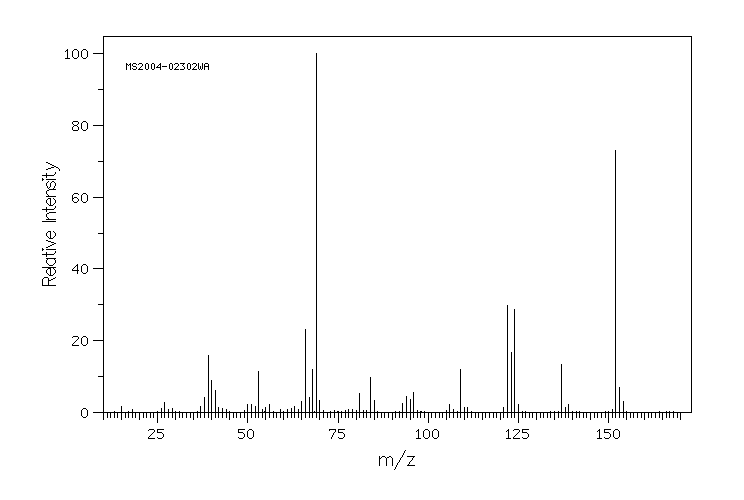
质谱MS
-
碳谱13CNMR
-
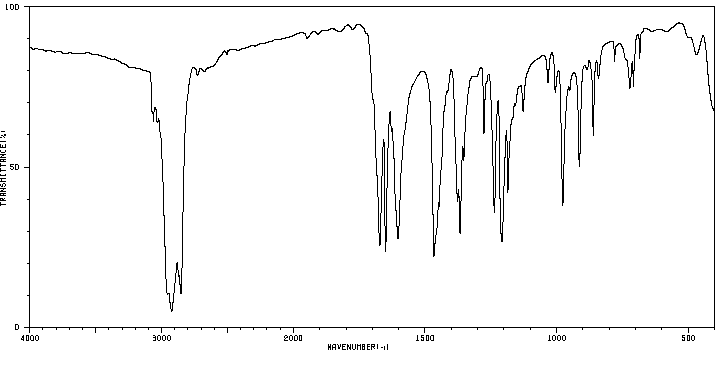
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷