2-羟基-4-正辛氧基二苯甲酮 | 1843-05-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:47-49 °C(lit.)
-
沸点:424.46°C (rough estimate)
-
密度:1.160g/cm3
-
闪点:102℃
-
溶解度:可溶于氯仿(轻微)、甲醇(轻微、超声处理)
-
LogP:7.360 (est)
-
物理描述:DryPowder; DryPowder, Liquid; OtherSolid; PelletsLargeCrystals
-
颜色/状态:Crystals
-
蒸汽压力:3.38X10-8 mm Hg at 20 °C (measured by transpiration method)
-
稳定性/保质期:
-
分解:Hazardous decomposition products formed under fire conditions. - Carbon oxides
-
汽化热:Enthalpy of vaporization: 102.1 kJ/mol
-
解离常数:pKa = 10.16 at 25 °C (Estimated with SPARC, OH-group)
计算性质
-
辛醇/水分配系数(LogP):6.8
-
重原子数:24
-
可旋转键数:10
-
环数:2.0
-
sp3杂化的碳原子比例:0.38
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xi,N
-
安全说明:S26,S36,S60,S61
-
危险类别码:R36/37/38,R50/53,R43
-
WGK Germany:1
-
海关编码:2914509090
-
危险品运输编号:UN 3077 9/PG 3
-
RTECS号:DJ1595000
-
包装等级:Z01
-
危险标志:GHS07
-
危险性描述:H317
-
危险性防范说明:P280
-
储存条件:1. 保持贮藏器密封,储存在阴凉、干燥的地方,并确保工作间有良好的通风或排气装置。 2. 该产品不易燃、不腐蚀,具有较好的储存稳定性。
SDS
| 第一部分:化学品名称 |
| 化学品中文名称: | 2-羟基-正辛氧基二苯甲酮 |
| 化学品英文名称: | 2-hydroxy-4-n-octoxy-benzophenone |
| 中文俗名或商品名: | |
| Synonyms: | |
| CAS No.: | 1843-05-6 |
| 分子式: | C 21 H 26 O 3 |
| 分子量: | 326 |
| 第二部分:成分/组成信息 |
| 纯化学品 混合物 | ||||||
| 化学品名称:2-羟基-正辛氧基二苯甲酮 | ||||||
|
| 第三部分:危险性概述 |
| 危险性类别: | |
| 侵入途径: | 无资料 |
| 健康危害: | 本品有刺激作用, 有麻醉作用。 |
| 环境危害: | 无资料 |
| 燃爆危险: | 本品可燃,具刺激性。 |
| 第四部分:急救措施 |
| 皮肤接触: | 脱去污染的衣着,用流动清水冲洗。 |
| 眼睛接触: | 无资料 |
| 吸入: | 脱离现场至空气新鲜处。如呼吸困难,给输氧。就医。 |
| 食入: | 饮足量温水,催吐。就医。 |
| 第五部分:消防措施 |
| 危险特性: | 遇明火、高热可燃。 |
| 有害燃烧产物: | 一氧化碳、二氧化碳。 |
| 灭火方法及灭火剂: | 消防人员须佩戴防毒面具、穿全身消防服,在上风向灭火。灭火剂:雾状水、泡沫、干粉、二氧化碳、砂土。 |
| 消防员的个体防护: | 无资料 |
| 禁止使用的灭火剂: | 无资料 |
| 闪点(℃): | 无资料 |
| 自燃温度(℃): | 无资料 |
| 爆炸下限[%(V/V)]: | 无资料 |
| 爆炸上限[%(V/V)]: | 无资料 |
| 最小点火能(mJ): | |
| 爆燃点: | |
| 爆速: | |
| 最大燃爆压力(MPa): | |
| 建规火险分级: |
| 第六部分:泄漏应急处理 |
| 应急处理: | 隔离泄漏污染区,限制出入。切断火源。建议应急处理人员戴防尘面具(全面罩),穿防毒服。用砂土、干燥石灰或苏打灰混合。避免扬尘,小心扫起,置于袋中转移至安全场所。若大量泄漏,用塑料布、帆布覆盖。收集回收或运至废物处理场所处置。 |
| 第七部分:操作处置与储存 |
| 操作注意事项: | 密闭操作,局部排风。操作人员必须经过专门培训,严格遵守操作规程。建议操作人员佩戴自吸过滤式防尘口罩,戴化学安全防护眼镜,穿防毒物渗透工作服,戴橡胶手套。远离火种、热源,工作场所严禁吸烟。使用防爆型的通风系统和设备。避免产生粉尘。避免与氧化剂接触。搬运时要轻装轻卸,防止包装及容器损坏。配备相应品种和数量的消防器材及泄漏应急处理设备。倒空的容器可能残留有害物。 |
| 储存注意事项: | 储存于阴凉、通风的库房。远离火种、热源。应与氧化剂分开存放,切忌混储。配备相应品种和数量的消防器材。储区应备有合适的材料收容泄漏物。 |
| 第八部分:接触控制/个体防护 |
| 最高容许浓度: | 无资料 |
| 监测方法: | 无资料 |
| 工程控制: | 密闭操作,局部排风。 |
| 呼吸系统防护: | 空气中粉尘浓度超标时,必须佩戴自吸过滤式防尘口罩。紧急事态抢救或撤离时,应该佩戴空气呼吸器。 |
| 眼睛防护: | 戴化学安全防护眼镜。 |
| 身体防护: | 穿防毒物渗透工作服。 |
| 手防护: | 戴橡胶手套。 |
| 其他防护: | 工作现场禁止吸烟、进食和饮水。工作完毕,淋浴更衣。保持良好的卫生习惯。 |
| 第九部分:理化特性 |
| 外观与性状: | 浅黄色结晶粉末。 |
| pH: | |
| 熔点(℃): | 48-49 |
| 沸点(℃): | 无资料 |
| 相对密度(水=1): | 1.16(25℃) |
| 相对蒸气密度(空气=1): | 无资料 |
| 饱和蒸气压(kPa): | |
| 燃烧热(kJ/mol): | |
| 临界温度(℃): | |
| 临界压力(MPa): | |
| 辛醇/水分配系数的对数值: | |
| 闪点(℃): | 无资料 |
| 引燃温度(℃): | 无资料 |
| 爆炸上限%(V/V): | 无资料 |
| 爆炸下限%(V/V): | 无资料 |
| 分子式: | C 21 H 26 O 3 |
| 分子量: | 326 |
| 蒸发速率: | |
| 粘性: | |
| 溶解性: | 不溶于水,溶于丙酮、苯、乙醇。 |
| 主要用途: | 作为紫外线吸收剂用于乙烯基树脂、聚苯乙烯、纤维素塑料、聚酯、聚酰胺等塑料、纤维及涂料中。 |
| 第十部分:稳定性和反应活性 |
| 稳定性: | 在常温常压下 |
| 禁配物: | 强氧化剂。 |
| 避免接触的条件: | 无资料 |
| 聚合危害: | 无资料 |
| 分解产物: | 无资料 |
| 第十一部分:毒理学资料 |
| 急性毒性: | 无资料 |
| 急性中毒: | 无资料 |
| 慢性中毒: | 无资料 |
| 亚急性和慢性毒性: | |
| 刺激性: | 无资料 |
| 致敏性: | |
| 致突变性: | |
| 致畸性: | |
| 致癌性: |
| 第十二部分:生态学资料 |
| 生态毒理毒性: | 无资料 |
| 生物降解性: | 无资料 |
| 非生物降解性: | 无资料 |
| 生物富集或生物积累性: |
| 第十三部分:废弃处置 |
| 废弃物性质: | 无资料 |
| 废弃处置方法: | 无资料 |
| 废弃注意事项: | 无资料 |
| 第十四部分:运输信息 |
| |
| 危险货物编号: | 无资料 |
| UN编号: | 无资料 |
| 包装标志: | |
| 包装类别: | |
| 包装方法: | 无资料 |
| 运输注意事项: | 起运时包装要完整,装载应稳妥。运输过程中要确保容器不泄漏、不倒塌、不坠落、不损坏。严禁与氧化剂、食用化学品等混装混运。运输途中应防曝晒、雨淋,防高温。车辆运输完毕应进行彻底清扫。 |
| RETCS号: | |
| IMDG规则页码: |
| 第十五部分:法规信息 |
| 国内化学品安全管理法规: | 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存、运输、装卸等方面均作了相应规定。 |
| 国际化学品安全管理法规: |
| 第十六部分:其他信息 |
| 参考文献: | |
| 填表时间: | 2007年12月31日 |
| 填表部门: | 无资料 |
| 数据审核单位: | 无资料 |
| 修改说明: | 无资料 |
| 其他信息: | |
| MSDS修改日期: | 1900年1月1日 |
制备方法与用途
概述
紫外线吸收剂UV-531属于二苯甲酮类,化学名为2-羟基-4-正辛氧基二苯甲酮。它常温下为淡黄色针状结晶粉末,是一种性能卓越的高效防老化助剂。能够强烈吸收波长在270~340nm范围内的紫外光,并具有色浅、无毒、相容性好、迁移性小、易于加工等特点。
应用
UV-531广泛应用于PE、PVC、PP、PS、PC、有机玻璃、丙纶纤维和乙烯醋酸乙烯酯等材料中。此外,它还能够为干性酚醛和醇酸清漆类、聚氨酯类、丙烯酸类、环氧类和其它空气干燥产品及汽车整修漆、粉末涂料、聚氨酯、橡胶制品提供良好的光稳定效果。建议用量为0.1%-0.5%。
特性
紫外线吸收剂UV-531对聚合物有卓越的保护作用,并有助于减少色泽,同时延缓材料泛黄并阻滞其物理性能损失。它与聚烯烃具有良好的相容性,低挥发性,在加工过程中不易流失,且不会影响制品气味。粉末和片状两种形式中,片状UV-531使用方便,环保,降低了加工难度。
理化性质外观
透光率
- 440nm ≥97.0%
- 500nm ≥98%
干燥失重
- ≤0.5%
灰 分
- ≤0.1%
熔 点
- 152-154℃
含 量
- ≥99%
毒 性
- LD 50 >2g/Kg(大白鼠经口)
| 外 观 | 淡黄色针状结晶粉末 | | 含 量 | ≥99% | | 熔 点 | 47-49°C | | 灰 份 | <0.1% | | 透光率(25℃) | - 360nm >80% - 340nm >70% - 320nm >60% - 300nm >50% - 280nm >40% - 260nm >30% | | 溶解性 | 易溶于苯、正己烷、丙酮,稍溶于乙醇,微溶于二氯乙烷 |
生产方法将2,4-二羟基二苯甲酮、1-溴正辛烷、丙酮和碳酸钾的混合物加热反应,过滤并浓缩。冷却后再次过滤并浓缩。最终产品通过乙醇重结晶获得UV-531。原料消耗定额为:2,4-二羟基二苯甲酮935kg/t、1-溴正辛烷1265kg/t、碳酸钾(95.5%)910kg/t、丙酮1040kg/t。
储存上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,4-二羟二苯甲酮 2,4-dihydroxybenzophenone 131-56-6 C13H10O3 214.221 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 紫外线吸收剂UV-9 Benzophenone-3 131-57-7 C14H12O3 228.247 —— 5,5'-methylene-bis(2-hydroxy-4-octoxybenzophenone) 69119-79-5 C43H52O6 664.882 —— (2-dichlorophosphoryloxy-4-octoxyphenyl)-phenylmethanone 129973-36-0 C21H25Cl2O4P 443.307
反应信息
-
作为反应物:描述:2-羟基-4-正辛氧基二苯甲酮 在 五氯化磷 作用下, 以 苯 为溶剂, 反应 2.5h, 以63%的产率得到(2-dichlorophosphoryloxy-4-octoxyphenyl)-phenylmethanone参考文献:名称:Intramolecular cyclization of 9,10-anthraquinones promoted by reaction with halogenophosphoranes摘要:DOI:10.1016/s0040-4020(01)86769-7
-
作为产物:描述:参考文献:名称:PANDIT, ANIL;PANDYA, SUBHASH, RES. AND IND., 32,(1987) N 3, 143-147摘要:DOI:
文献信息
-
PHOTOPROTECTIVE COMPOSITIONS COMPRISING PHOTOSENSITIVE 1,3,5-TRIAZINE COMPOUNDS, DIBENZOYLMETHANE COMPOUNDS AND SILICEOUS S-TRIAZINES SUBSTITUTED WITH TWO AMINOBENZOATE OR AMINOBENZAMIDE GROUPS申请人:L'OREAL公开号:US20170135933A1公开(公告)日:2017-05-18UV-photoprotective, topically applicable cosmetic/dermatological compositions contain: (a) at least one dibenzoylmethane compound, (b) at least one 1,3,5-triazine compound that is photosensitive in the presence of a dibenzoylmethane compound, and (c) at least one siliceous s-triazine compound substituted with two aminobenzoate or aminobenzamide groups, or a tautomeric form thereof, the 1,3,5-triazine compounds being improvedly photostable in such compositions.
-
Synthesis and Reactivity of α‐Cumyl Bromodifluoromethanesulfenate: Application to the Radiosynthesis of [ <sup>18</sup> F]ArylSCF <sub>3</sub>作者:Jiang Wu、Qunchao Zhao、Thomas C. Wilson、Stefan Verhoog、Long Lu、Véronique Gouverneur、Qilong ShenDOI:10.1002/anie.201813708日期:2019.2.18A highly reactive electrophilic bromodifluoromethylthiolating reagent, α-cumyl bromodifluoro-methanesulfenate 1, was prepared to allow for direct bromodifluoromethylthiolation of aryl boron reagents. This coupling reaction takes place under copper catalysis, and affords a large range of bromodifluoromethylthiolated arenes. These compounds are amenable to various transformations including halogen exchange
-
Heterocyclic Compound申请人:Amasaki Ichiro公开号:US20100004439A1公开(公告)日:2010-01-07A compound represented by the following Formula (1): wherein, Het 1 represents a bivalent five- or six-membered aromatic heterocyclic residue and may further be substituted; X a to X d each independently represent a heteroatom and may further be substituted; Y a to Y f each independently represent a heteroatom or a carbon atom and may further be substituted; the ring bound to Het 1 may have a double bond at any position以下是您提供的化学公式(1)的中文翻译: 其中,Het1代表一个二价的五元或六元芳香杂环基团,且可以进一步被取代;Xa至Xd每个独立地代表一个杂原子,且可以进一步被取代;Ya至Yf每个独立地代表一个杂原子或一个碳原子,且可以进一步被取代;与Het1相连的环可以在任何位置有一个双键。
-
[EN] HEMI-AMINAL ETHERS AND THIOETHERS OF N-ALKENYL CYCLIC COMPOUNDS<br/>[FR] ÉTHERS ET THIOÉTHERS HÉMIAMINAUX DE COMPOSÉS CYCLIQUES N-ALCÉNYLIQUES申请人:ISP INVESTMENTS INC公开号:WO2014116560A1公开(公告)日:2014-07-31Described herein are hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds that may be produced through a reaction comprising: (A) at least one first reactant represented by a structure (I), wherein X is a functionalized or unfunctionalized C1-C5 alkylene group optionally having one or more heteroatoms, and each R1, R2, and R3 is independently selected from the group consisting of hydrogen and functionalized and unfunctionalized alkyl groups optionally having one or more heteroatoms, and (B) at least one second reactant having at least one hydroxyl moiety or thiol moiety. The hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds may comprise a polymerizable moiety, in which case they may be left as-is or used to create homopolymers or non-homopolymers, or they may not comprise a polymerizable moiety. A wide variety of formulations may be created using the hemi-aminal ethers and thioethers of N-alkenyl cyclic compounds, including personal care, oilfield, and construction formulations.
-
Extracts of Isochrysis sp.申请人:Herrmann Martina公开号:US20100080761A1公开(公告)日:2010-04-01The present invention relates to extracts of Isochrysis sp., preferably Tahitian Isochrysis, its cosmetic, dermatological and/or therapeutic uses and compositions and cosmetic, dermatological or therapeutic products comprising such an extract of Isochrysis sp., preferably Tahitian Isochrysis.
表征谱图
-
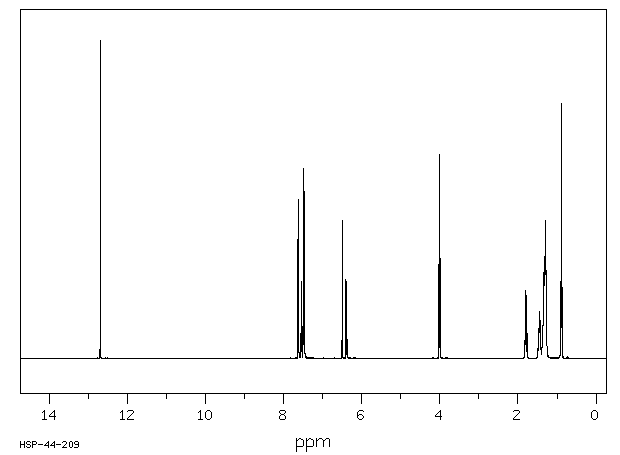
氢谱1HNMR
-
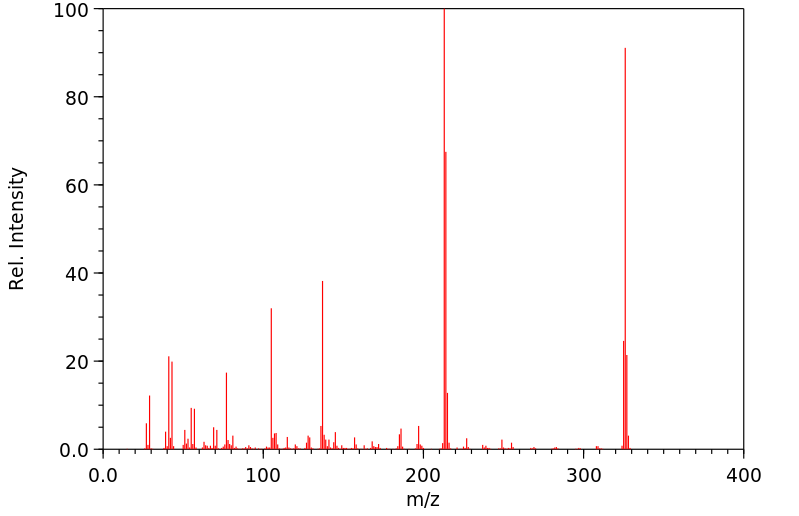
质谱MS
-
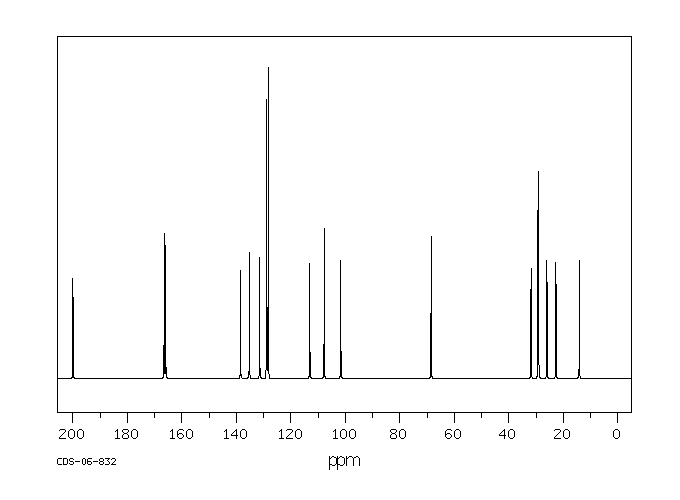
碳谱13CNMR
-
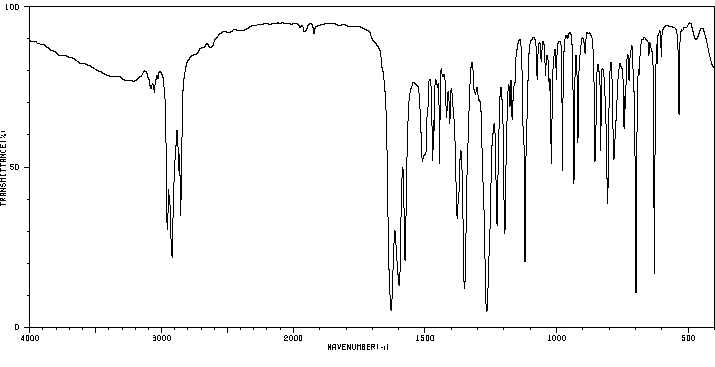
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息