2-羟基-4-甲基嘧啶盐酸盐
中文名称
2-羟基-4-甲基嘧啶盐酸盐
中文别名
4-甲基嘧啶-2-醇盐酸盐;四甲基嘧啶-2-醇盐酸盐;2-羟基-4-甲基嘧啶盐酸
英文名称
2-Hydroxy-4-methylpyrimidine hydrochloride
英文别名
hydron;6-methyl-1H-pyrimidin-2-one;chloride
CAS
——
化学式
C5H6N2O*ClH
mdl
——
分子量
146.576
InChiKey
FDRYQDWXFZBBAD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.5
-
重原子数:9
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:41.5
-
氢给体数:2
-
氢受体数:1
反应信息
-
作为反应物:描述:2-羟基-4-甲基嘧啶盐酸盐 在 水 、 溶剂黄146 、 sodium nitrite 作用下, 以94%的产率得到2-oxo-1,2-dihydro-pyrimidine-4-carbaldehyde oxime参考文献:名称:NOVEL OXAZOLE DERIVATIVES THAT INHIBIT SYK摘要:本发明涉及替代噁唑衍生物,能选择性地调节、调控和/或抑制由某些涉及人类和动物疾病的特定天然和/或突变蛋白激酶介导的信号转导,如细胞增殖、代谢、自身免疫、过敏、血液学、炎症和退行性疾病。具体来说,本发明的化合物是Syk抑制剂。本发明还涉及一种制造本发明化合物的方法。公开号:EP3144307A1
-
作为产物:描述:参考文献:名称:Lee, Thomas W. S.; Rettig, Steven J.; Stewart, Ross, Canadian Journal of Chemistry, 1984, vol. 62, p. 1194 - 1202摘要:DOI:
文献信息
-
THERAPEUTICALLY ACTIVE COMPOUNDS AND THEIR METHODS OF USE申请人:Lemieux Rene M.公开号:US20150031627A1公开(公告)日:2015-01-29Provided are methods of treating a cancer characterized by the presence of a mutant allele of IDH1/2 comprising administering to a subject in need thereof a compound described here.提供的方法是治疗一种由IDH1/2突变等位基因特征性存在的癌症,包括向需要此治疗的患者施用此处描述的化合物。
-
[EN] THERAPEUTICALLY ACTIVE COMPOUNDS AND THEIR METHODS OF USE<br/>[FR] COMPOSÉS THÉRAPEUTIQUEMENT ACTIFS ET LEURS MÉTHODES D'UTILISATION申请人:AGIOS PHARMACEUTICALS INC公开号:WO2015010297A1公开(公告)日:2015-01-29Provided are methods of treating a cancer characterized by the presence of a mutant allele of IDH1/2 comprising administering to a subject in need thereof a compound described here.提供的方法是治疗一种由IDH1/2突变等位基因特征性存在的癌症,包括向需要此治疗的患者施用此处描述的化合物。
-
Conversion of 1-benzyl-4-aminotetrahydropyridine-3-carboxylic acid methyl ester to antithrombotic pyrido[4,3-<i>d</i>]pyrimidine-2,4-diones and to (2-oxotetrahydropyrimidin-4-ylidene)acetic acid methyl esters作者:H. Furrer、R. Wagner、H.-W. FehlhaberDOI:10.1002/jhet.5570310649日期:1994.11two methods. The thermal fusion of 1 with ureas gave the pyrido[4,3-d]-pyrimidine-2,4-diones 5a, 2 and 5b and unexpectedly the esters 6 and 7. The structure of 6 was deduced from its spectroscopic properties and was proven by ozonolysis to cleavage products 9 and 10 and by oxidative hydrolysis to pyrimidin-2-one 13. The (Z)-configured 6 was converted to (E)-configured 7 by methylation. The products 5a
-
[EN] THERAPEUTICALLY ACTIVE COMPOUNDS AND THEIR METHODS OF USE<br/>[FR] COMPOSÉS THÉRAPEUTIQUEMENT ACTIFS ET LEURS PROCÉDÉS D'UTILISATION申请人:AGIOS PHARMACEUTICALS INC公开号:WO2013107291A1公开(公告)日:2013-07-25Provided are methods of treating a cancer characterized by the presence of a mutant allele of IDH1/2 comprising administering to a subject in need thereof a compound described here.提供的方法是治疗一种由IDH1/2突变等位基因特征性存在的癌症,包括向需要此治疗的患者施用此处描述的化合物。
-
[EN] THERAPEUTICALLY ACTIVE COMPOUNDS AND USE THEREOF<br/>[FR] COMPOSÉS THÉRAPEUTIQUEMENT ACTIFS ET UTILISATION DE CEUX-CI申请人:AGIOS PHARMACEUTICALS INC公开号:WO2015010626A1公开(公告)日:2015-01-29Provided are therapeutically active compounds and the use in manufacture of medicaments for treating a cancer characterized by the presence of a mutant allele of IDH1.提供的是治疗上有效的化合物及其在制造用于治疗具有IDH1突变等位基因的癌症的药物中的应用。
表征谱图
-
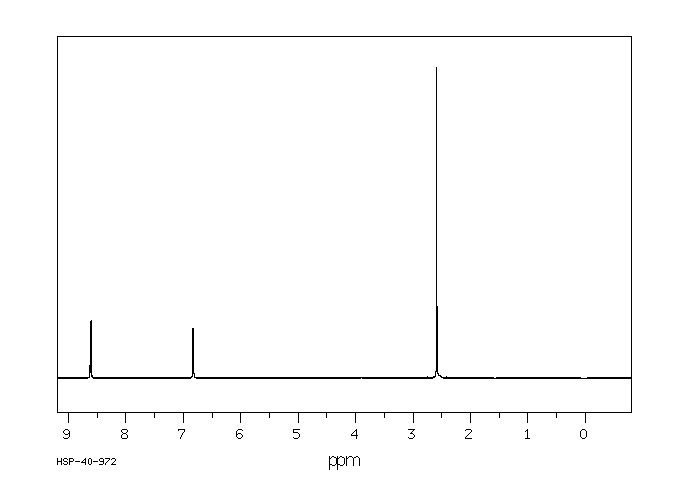
氢谱1HNMR
-
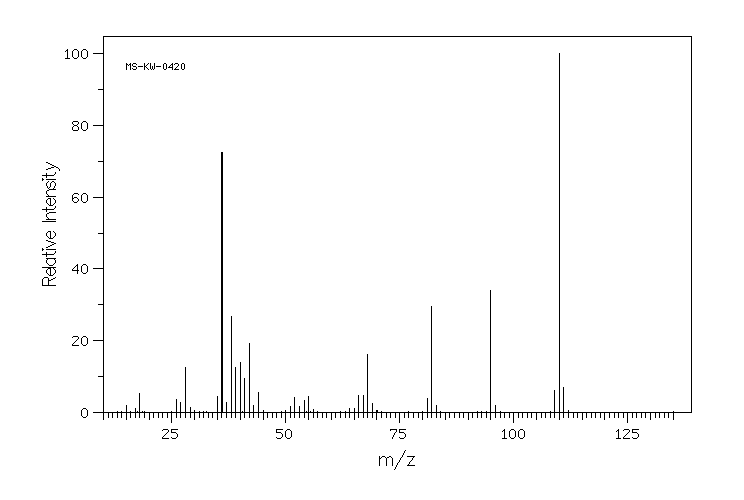
质谱MS
-
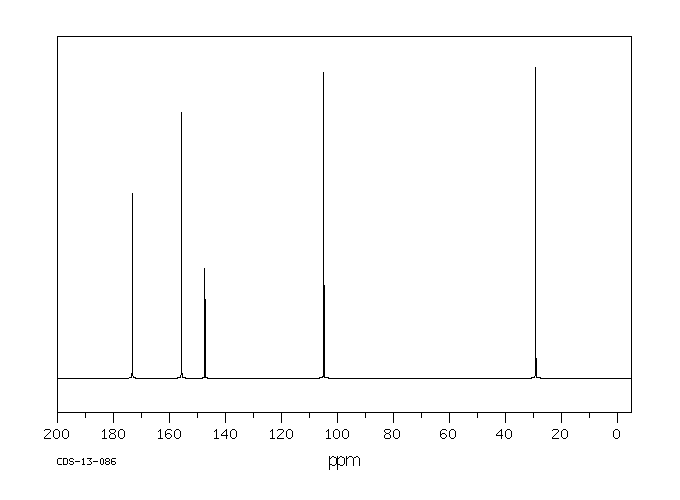
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3