2-苯基-薁 | 19227-07-7
中文名称
2-苯基-薁
中文别名
薁,2-苯基-
英文名称
2-phenylazulene
英文别名
2-Phenyl-azulen;Azulene, 2-phenyl-
CAS
19227-07-7
化学式
C16H12
mdl
——
分子量
204.271
InChiKey
VUKMMANDBUENJS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.5
-
重原子数:16
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902909090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 奥苷菊环 azulene 275-51-4 C10H8 128.174
反应信息
-
作为反应物:描述:参考文献:名称:2,2'-Diaryl-1,1'-Biazulenes的立体化学,立体动力学,氧化还原和络合行为。摘要:合成了2,2'-二芳基-1,1'-二氮杂烯,并基于氧化还原测量结果提出了氮杂亚基之间的电子通讯。在1位上的azulene的连接也似乎增加了HOMO水平。另外,对2-芳基azulenes的循环伏安法测量显示了与氧化有关的返回峰,而对于azulene则没有观察到。单电子氧化剂的稳定化可能归因于SOMO-HOMO能量转化现象。氮杂二聚体的X射线晶体学分析表明该物种具有同型结构,其中2-位的两个芳基均形成π-堆叠。对于该晶胞中的所有分子而言,该扭曲结构被表示为(R)-或(S)-构型。还显示出自发的分辨率。此外,从固体圆二色性(CD)光谱测量中,确定了分子的绝对构型与CD光谱之间的关系。外消旋旋转势垒约为 计算出27kcal mol-1。而且,吡啶基azulene二聚体在与PdCl 2反应时环化以形成3∶3配合物,其中联氮杂双环单元环化以得到(R)-和(S)-形式之间的比率为2∶1或1∶2。DOI:10.1002/cplu.201900262
-
作为产物:描述:参考文献:名称:Porshnev,Yu.N. et al., Journal of Organic Chemistry USSR (English Translation), 1974, vol. 10, p. 887 - 888摘要:DOI:
文献信息
-
Direct synthesis of 2-arylazulenes by [8+2] cycloaddition of 2<i>H</i>-cyclohepta[<i>b</i>]furan-2-ones with silyl enol ethers作者:Taku Shoji、Shuhei Sugiyama、Yoshiaki Kobayashi、Akari Yamazaki、Yukino Ariga、Ryuzi Katoh、Hiroki Wakui、Masafumi Yasunami、Shunji ItoDOI:10.1039/c9cc09376a日期:——We developed a procedure for the direct synthesis of 2-arylazulenes, which were obtained in moderate to excellent yields, by [8+2] cycloaddition of 2H-cyclohepta[b]furan-2-ones with aryl-substituted silyl enol ethers. The structures of some 2-arylazulenes were clarified by single-crystal X-ray analysis. The 2-phenylazulene derivatives obtained by this study showed noticeable fluorescence in acidic
-
Carboxylic Acid Directed C–H Arylation of Azulene作者:Zhuang Mao Png、Teck Lip Dexter Tam、Jianwei XuDOI:10.1021/acs.orglett.0c01576日期:2020.7.2example of directed C–H activation for azulene is reported. A variety of 2-arylazulenes are obtainable exclusively from 1-azulene carboxylic acids, with yields of up to 82%. Some heteroaryl groups such as pyridine and thiophene are also tolerated in the reaction. The efficacy of the reaction is found to be highly dependent on the conditions, with a phosphate base and a bulky carboxylic acid being key
-
First synthesis of 1-(indol-2-yl)azulenes by the Vilsmeier–Haack type arylation with triflic anhydride as an activating reagent作者:Taku Shoji、Yuta Inoue、Shunji ItoDOI:10.1016/j.tetlet.2012.01.044日期:2012.3Azulene derivatives reacted with 2-indolinones in the presence of triflic anhydride (Tf2O) to afford 1-(indol-2-yl)azulenes in good yields. In the cases of the reaction of 6-tert-butyl-1-(methylthio)azulene (11) and 1-(1,4-dihydropyridin-4-yl)azulene 14, 1,1′-biazulene derivative 24 and 1-(indol-2-yl)azulene (2) were obtained under the similar reaction conditions, respectively, instead of the presumed
-
A New Efficient Route to 2-Substituted Azulenes Based on Sulfonyl Group Directed Lithiation作者:Toshihisa Shibasaki、Takeo Ooishi、Nobuhiko Yamanouchi、Toshihiro Murafuji、Kei Kurotobi、Yoshikazu SugiharaDOI:10.1021/jo801166f日期:2008.10.17ring closure at the 8-position. 2-(Phenylsulfanyl)-1-azulenyl p-tolyl sulfone (2b) suffered from desulfonylation to form 2-phenylsulfanylazulene (4). The Suzuki coupling reaction of 2-iodo-1-azulenyl p-tolyl sulfone (2d) with arylboronic acids followed by desulfonylation efficiently gave 2-arylazulenes 10.
-
Synthesis and Properties of Fluoroazulenes. II. Electrophilic Fluorination of Azulenes with<i>N</i>-Fluoro Reagents作者:Tetsuya Ueno、Haruhiko Toda、Masafumi Yasunami、Masaaki YoshifujiDOI:10.1246/bcsj.69.1645日期:1996.63-difluoroazulenes were synthesized for the first time by the electrophilic fluorination of azulenes with N-fluoro reagents. Selective preparation of 1-fluoroazulenes were performed by the fluorination of methyl azulene-1-carboxylates, followed by demethoxycarbonylation in 100% H3PO4. 2-Substituted azulenes were fluorinated in higher yields. In the 1H NMR of 1-fluoroazulene, long-range JFH values were
表征谱图
-
氢谱1HNMR
-
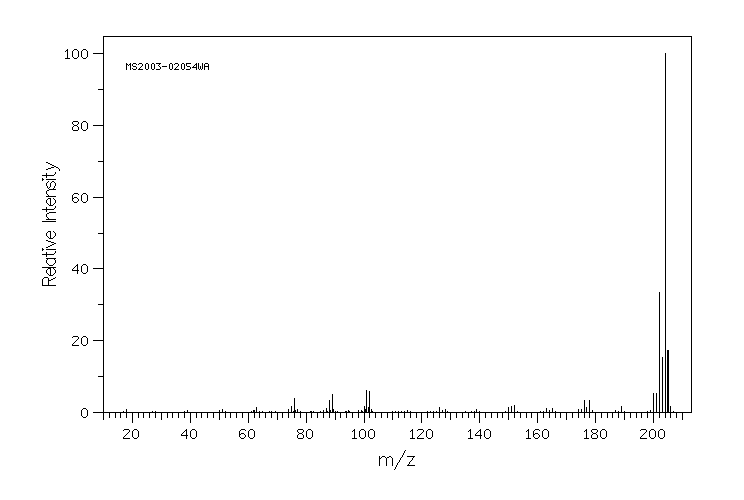
质谱MS
-
碳谱13CNMR
-
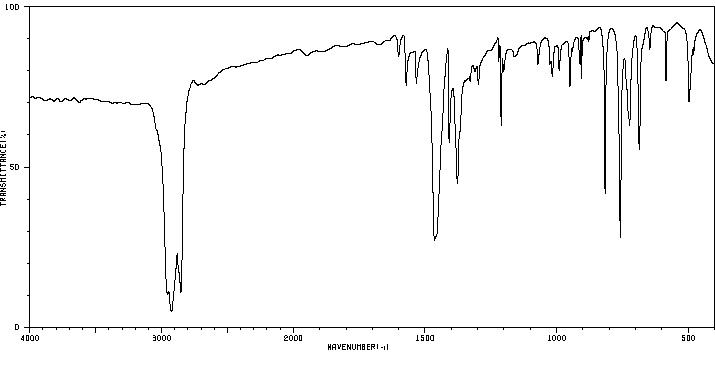
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-