3',6'-双(二乙氨基)-2-(4-硝基苯基)螺[异吲哚-1,9'-氧杂蒽]-3-酮 | 29199-09-5
中文名称
3',6'-双(二乙氨基)-2-(4-硝基苯基)螺[异吲哚-1,9'-氧杂蒽]-3-酮
中文别名
2,2'-双(二苯基磷)联苯
英文名称
3',6'-bis-diethylamino-2-(4-nitro-phenyl)-spiro[isoindoline-1,9'-xanthen]-3-one
英文别名
3',6'-Bis-diaethylamino-2-(4-nitro-phenyl)-spiro[isoindolin-1,9'-xanthen]-3-on;3',6'-bis(diethylamino)-2-(4-nitrophenyl)spiro[1H-isoindole-1,9'-[9H]xanthene]-3(2H)-one;3',6'-Bis(diethylamino)-2-(4-nitrophenyl)spiro(1H-isoindole-1,9'-(9H)xanthene)-3(2H)-one;3',6'-bis(diethylamino)-2-(4-nitrophenyl)spiro[isoindole-3,9'-xanthene]-1-one
CAS
29199-09-5
化学式
C34H34N4O4
mdl
——
分子量
562.668
InChiKey
XZXFZILEZWXEND-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:197.0 to 201.0 °C
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会分解,也未有已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):6.8
-
重原子数:42
-
可旋转键数:7
-
环数:6.0
-
sp3杂化的碳原子比例:0.26
-
拓扑面积:81.8
-
氢给体数:0
-
氢受体数:6
安全信息
-
海关编码:2934999090
SDS
Section I.Chemical Product and Company Identification
Chemical Name 3',6'-Bis(diethylamino)-2-(4-nitrophenyl)
spiro[isoindole-1,9'-xanthene]-3-one
Portland OR
Synonym Spiro[1H-isoindole-1,9'-[9H]xanthen]-3(2H)-one,
3',6'-bis(diethylamino)-2-(4-nitrophenyl)- (CA INDEX
NAME)
Chemical Formula C34H34N4O4
CAS Number 29199-09-5
Section II. Composition and Information on Ingredients
Toxicology Data
Chemical Name CAS Number Percent (%) TLV/PEL
Min. 97.0 (T) Not available. Not available.
3',6'-Bis(diethylamino)-2-(4-nitrophenyl) 29199-09-5
spiro[isoindole-1,9'-xanthene]-3-one
Section III. Hazards Identification
Acute Health Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
Inhalation If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Ingestion
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic
material was ingested; the absence of such signs, however, is not conclusive.
Section V. Fire and Explosion Data
Not available.
Flammability May be combustible at high temperature. Auto-Ignition
Flash Points Not available. Flammable Limits Not available.
Combustion Products These products are toxic carbon oxides (CO, CO2), nitrogen oxides (NO, NO2).
Fire Hazards
Not available.
Risks of explosion of the product in presence of mechanical impact: Not available.
Explosion Hazards
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Consult with local fire authorities before attempting large scale fire-fighting operations.
Continued on Next Page
3',6'-Bis(diethylamino)-2-(4-nitrophenyl)
spiro[isoindole-1,9'-xanthene]-3-one
Section VI. Accidental Release Measures
Spill Cleanup Use a shovel to put the material into a convenient waste disposal container. Finish cleaning the spill by rinsing any
contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
Instructions
disposal.
Section VII. Handling and Storage
Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the container and store in a dry, cool
Handling and Storage
place. Avoid excessive heat and light. Do not breathe dust.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended
exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants
below the exposure limit.
Personal Protection Splash goggles. Lab coat. Dust respirator. Boots. Gloves. Suggested protective clothing might not be sufficient; consult a
specialist BEFORE handling this product. Be sure to use a MSHA/NIOSH approved respirator or equivalent.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solid. (Yellowish-red small granular solid.) Solubility
Physical state @ 20°C Not available.
Not available.
Specific Gravity
Molecular Weight 562.66 Partition Coefficient Not available.
Boiling Point Not available. Vapor Pressure Not applicable.
198°C (388.4°F) Not available.
Melting Point Vapor Density
Not available. Volatility Not available.
Refractive Index
Not available.
Critical Temperature Not available. Odor
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
RTECS Number Not available.
Eye Contact. Ingestion. Inhalation.
Routes of Exposure
Toxicity Data Not available.
CARCINOGENIC EFFECTS : Not available.
Chronic Toxic Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Acute Toxic Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling this
compound.
Section XII. Ecological Information
Not available.
Ecotoxicity
Environmental Fate Not available.
Continued on Next Page
3',6'-Bis(diethylamino)-2-(4-nitrophenyl)
spiro[isoindole-1,9'-xanthene]-3-one
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a
Waste Disposal
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
Not applicable.
PIN Number
Proper Shipping Name Not applicable.
Not applicable.
Packing Group (PG)
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not controlled under WHMIS (Canada).
(Canada)
EINECS Number (EEC) 249-501-8
EEC Risk Statements Not available.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:参考文献:名称:Substituent effects on the turn-on kinetics of rhodamine-based fluorescent pH probes摘要:基于罗丹明螺内酰胺(RSL)结构的荧光开启探针近来已成为检测 pH 值、金属离子和其他相关分析物的常用方法。RSL 是无色无荧光的,直到目标分析物诱导螺环系统打开,露出完全共轭的高荧光罗丹明染料。在被酸打开的 RSL 中,我们观察到荧光开启过程的动力学差异很大,因此有些探针在需要快速读数或 pH 值随时间波动的情况下无法使用。在此,我们对 RSL 的荧光开启动力学进行了系统研究,以探索反应速率受螺内酰胺环系统电子特性影响的假设。研究人员利用罗丹明 B 制备了一系列 8 种苯胺衍生的 RSL,这些 RSL 的对位取代基的范围从给电子到抽电子不等。这种效应的原因是取代基破坏了反应中间体的稳定性。随着各系列反应速率的增加,荧光强度也随之增加。这一结果归因于染料荧光形式浓度的增加,并与该体系的预期平衡特性相一致。这些发现被应用于设计反应更快、荧光更强的 RSL pH 探针。DOI:10.1039/c3ob42089b
-
作为产物:描述:参考文献:名称:Selective sensing of Al3+ ions by nitrophenyl induced coordination: imaging in zebrafish brain tissue摘要:通过硝基苯诱导的配位作用,已经开发并应用了一种对Al3+离子的选择性感测方法,用于检测斑马鱼脑组织中的Al3+。DOI:10.1039/c9ob00460b
文献信息
-
Palladium‐Catalyzed Methylation of Nitroarenes with Methanol作者:Lin Wang、Helfried Neumann、Matthias BellerDOI:10.1002/anie.201814146日期:2019.4.8procedure for the synthesis of N‐methyl‐arylamines directly from nitroarenes using methanol as green methylating agent was developed. The key to success is the use of a specific catalyst system consisting of palladium acetate and the ligand 1‐[2,6‐bis(isopropyl)phenyl]‐2‐[tert‐butyl(2‐pyridinyl)phosphino]‐1H‐Imidazole (L1). The generality of this protocol is demonstrated in the synthesis of more than 20
-
Photocatalytic Water‐Splitting Coupled with Alkanol Oxidation for Selective <i>N</i> ‐alkylation Reactions over Carbon Nitride作者:Yangsen Xu、Zhaofei Zhang、Chuntian Qiu、Shaoqin Chen、Xiang Ling、Chenliang SuDOI:10.1002/cssc.202002459日期:2021.1.21(PWST) enables the direct use of water as appealing “liquid hydrogen source” for transfer hydrogenation reactions. Currently, the development of PWST‐based transfer hydrogenations is still in an embryonic stage. Previous reports generally centered on the rational utilization of the in situ generated H‐source (electrons) for hydrogenations, in which photogenerated holes were quenched by sacrificial光催化水分解技术(PWST)可以将水直接用作转移加氢反应的吸引人的“液态氢源”。目前,基于PWST的转移氢化的开发仍处于萌芽阶段。以前的报道通常集中在合理利用原位生成的氢源(电子)进行氢化的过程中,其中通过牺牲试剂淬灭了光生空穴。本文介绍了光还原氮在水分解过程中对液体H源和孔的充分利用硝基芳族化合物的烷基化。在该集成系统中,将水分解过程中产生的H物种设计用于还原硝基芳烃以生成胺,而烷醇则被孔氧化以使苯胺以及生成的仲胺进行级联烷基化。在广泛范围内获得的50多个示例证明了这种温和且可持续的耦合方法的普遍适用性。通过胺的选择性N-烷基化合成现有药物,进一步证明了该方案的合成效用。这种基于可持续水分解技术的策略突出了选择性合成有价值的氮的重要途径。由硝基芳烃和胺与水和链烷醇一起烷基化的精细化学品和药物。
-
Selective mono-N-methylation of nitroarenes with methanol catalyzed by atomically dispersed NHC-Ir solid assemblies作者:Jiaquan Wang、Jiajie Wu、Zhe-Ning Chen、Daheng Wen、Jiangbo Chen、Qingshu Zheng、Xin Xu、Tao TuDOI:10.1016/j.jcat.2020.06.004日期:2020.9dispersed active Ir(I) centers and the large π-conjugation rings endow the solid catalysts with an exceptionally high activity and selectivity for a broad substrate scope. Such solid NHC-Ir coordination assemblies are robust, which can be easily recovered and reused more than 10 runs without significant loss of their catalytic activity and selectivity. When combined with a subsequent formylation using the same
-
THERMOCHROMIC PIGMENT COMPOSITIONS申请人:SOCIETE BIC公开号:US20200207699A1公开(公告)日:2020-07-02A thermochromic pigment composition including compounds of formula (I): in which: X represents CHR 2 , O, OCO or CH═CH; Y represents O, COO, or OCOO; R 1 represents H or (CH 2 ) p CH 3 ; R 2 represents phenyl or H; m=12-18; n=0-14; p=12-18; and on the condition that, if n=0, X represents CHR 2 or CH═CH. The thermochromic pigment compositions having compounds of formula (I), include thermochromic pigment microcapsules. The thermochromic pigment capsules are useable for thermochromic pigment compositions in ink which may be used writing instruments.
-
[EN] DIESTER OF BISPHENOL FLUORENE COMPOUNDS AND THERMOCHROMIC PIGMENT COMPOSITIONS COMPRISING THE SAME<br/>[FR] DIESTER DE COMPOSÉS DE BISPHÉNOL FLUORÈNE ET COMPOSITIONS DE PIGMENTS THERMOCHROMIQUES COMPRENANT CELUI-CI申请人:SOCIÉTÉ BIC公开号:WO2020064537A1公开(公告)日:2020-04-02The present invention relates to the use of a compound represented by formula (I), wherein R1 and R2, identical or different, represent a C2-C30 alkyl group, a C2-C30 alkenyl group, a C2-C30 alkynyl group, or a C2-C30 alkoxy group, said alkyl, alkenyl, alkynyl, or alkoxy groups being optionally substituted with at least one hydroxy, halogen, amino, C1-C8 alkyl, or C1-C8 alkoxy group, as a temperature change regulating agent in a thermochromic ink composition. The present invention also relates to thermochromic pigment microcapsules comprising a compound of formula (I) according to the invention, to ink compositions comprising such thermochromic pigment microcapsules, and to writing instruments comprising such ink compositions. Finally, the invention also relates to a specific compound of formula (II) as such.
表征谱图
-
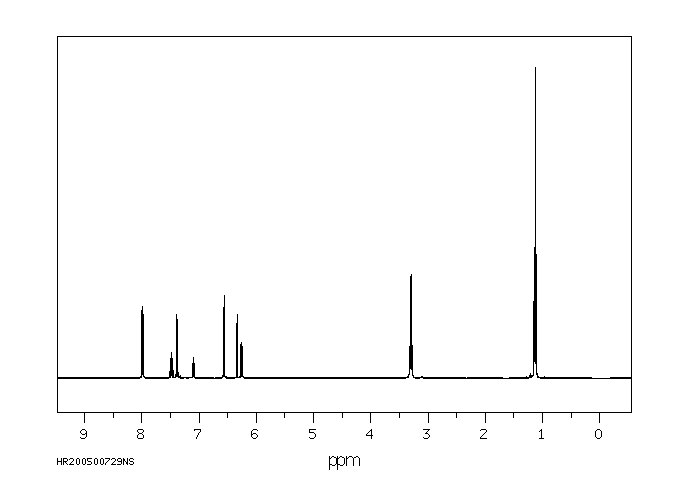
氢谱1HNMR
-
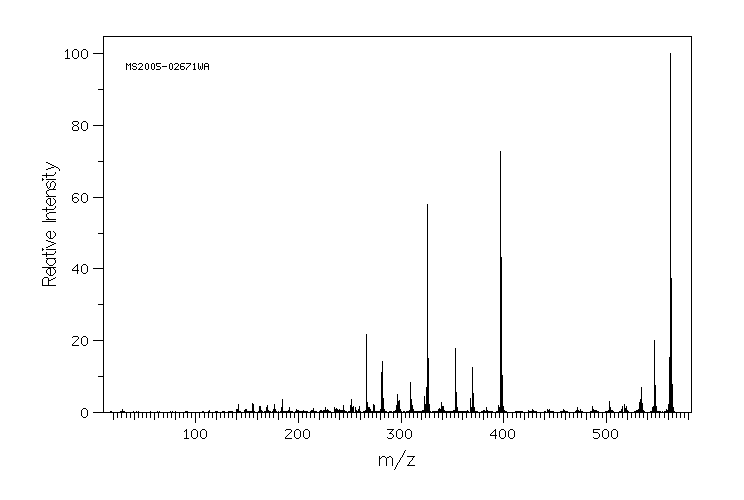
质谱MS
-
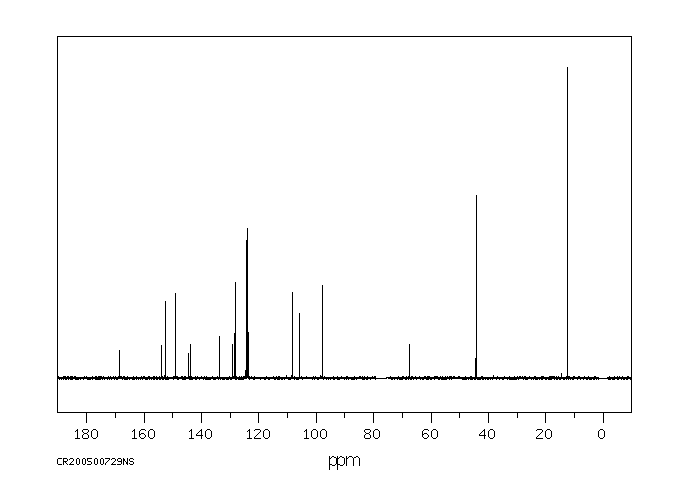
碳谱13CNMR
-
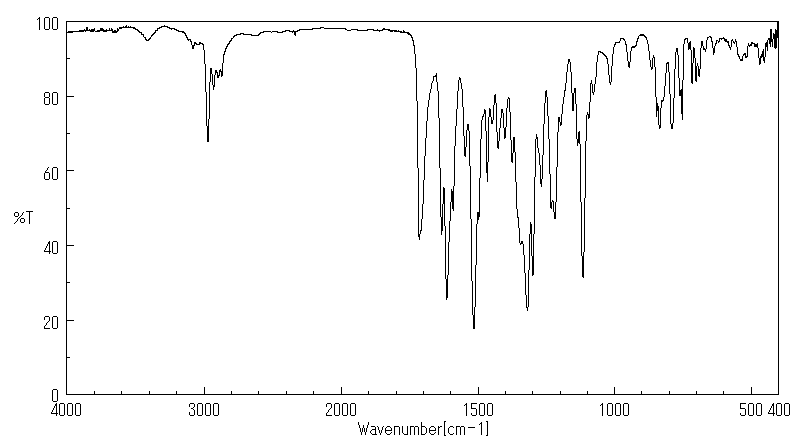
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-8-氟苯并二氢吡喃-4-胺
(2S)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
(2R)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
龙胆根素
龙胆SHAN酮
齿阿米素
黑色素-1
黄天精
麥角黃酮酸
鲍迪木醌
高邻苯二甲酸酐
高芒果苷
高氯酸罗丹明640
马佐卡林
香豆素-340
香豆素 339
食用色素红色105号
颜料红90:1铝色淀[CI45380:3]
颜料红172铝色淀[CI45430:1]
雏菊叶龙胆酮
降阿赛里奥; 1,3,6,7-四羟基氧杂蒽酮
阿米醇
阿米凯林
阿米凯林
阿扎那托
阿巴哌酮
阿尼地坦
阿尼地坦
阿匹氯铵
锌离子荧光探针-4
锆(2+)二[2-(2,4,5,7-四溴-3,6-二羟基氧杂蒽-9-基)-3,4,5,6-四氯苯甲酸酯]
铁力木呫吨酮-B
铁力木吨酮A
钠6'-羟基-5-[2-[4-(2-甲基-3-氧代-7H-咪唑并[1,2-d]吡嗪-6-基)苯氧基]乙基硫代氨基甲酰氨基]-3-氧代螺[2-苯并呋喃-1,9'-氧杂蒽]-3'-醇
钠4-{[6'-(二乙基氨基)-3'-羟基-3-氧代-3H-螺[2-苯并呋喃-1,9'-氧杂蒽]-2'-基]偶氮}-3-羟基-1-萘磺酸酯
钠2-(2,7-二氯-9H-氧杂蒽-9-基)苯甲酸酯
钙黄绿素乙酰甲酯
钙荧光探针Fluo-8,AM
钙荧光探针Fluo-4,AM
钙离子荧光探针
钙柑子
采木(HAEMATOXYLONCAMPECHIANUM)木质提取物
酸性媒介桃红3BM
邻苯三酚红
远志山酮III
转移核糖核酸(面包酵母)
赤藓红B异硫氰酸酯异构体II
赤藓红
诺大麻
西伯尔链接剂