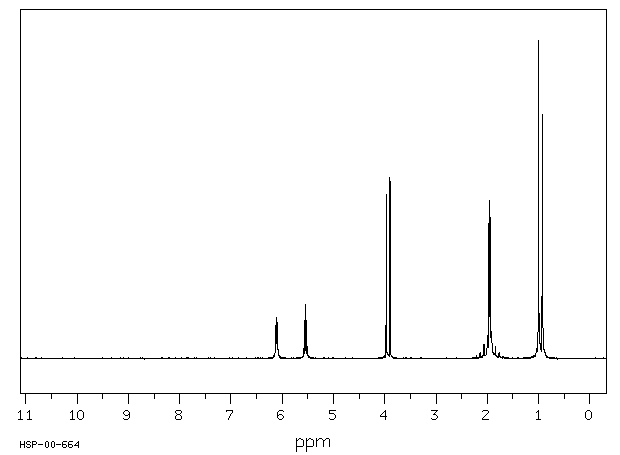
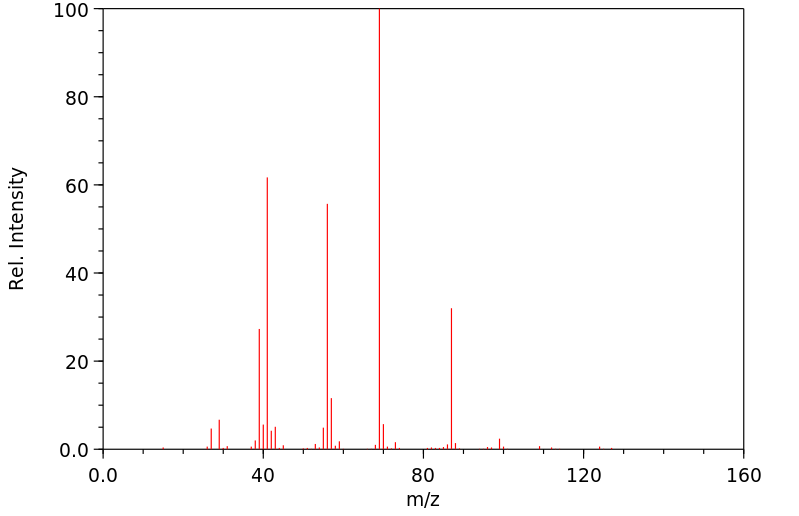
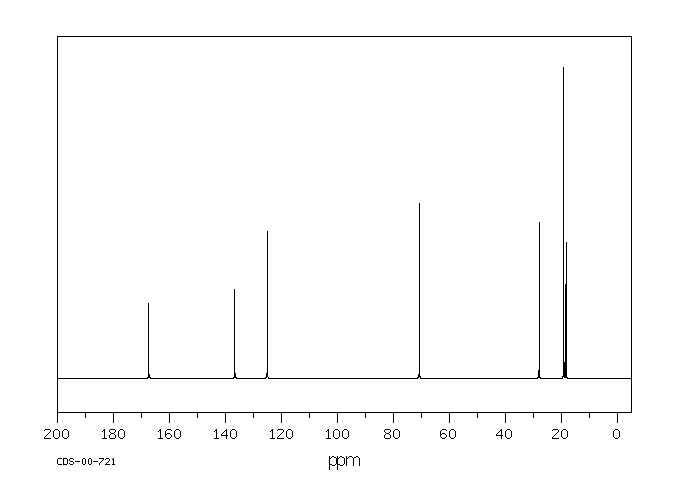
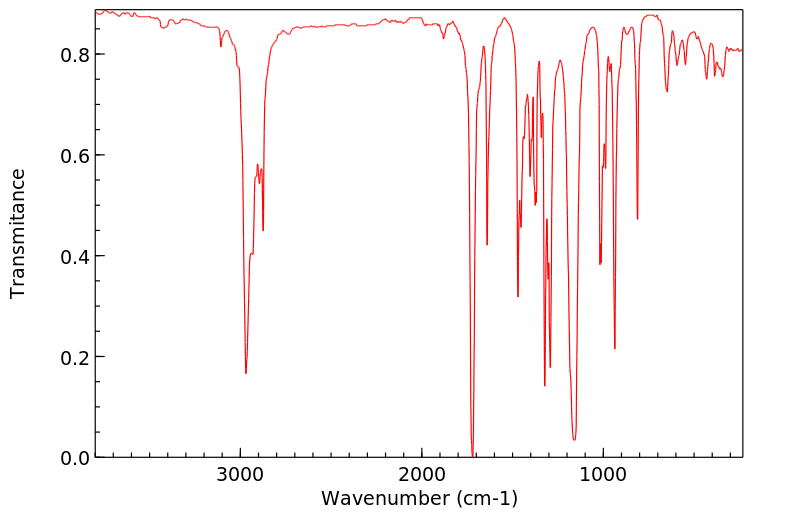
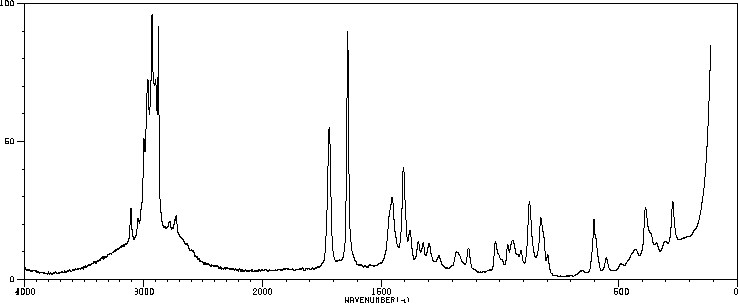
代谢
Methyl methacrylate and other short chain alkyl-methacrylate esters are initially hydrolyzed by non-specific carboxylesterases to methacrylic acid and the structurally corresponding alcohol in several tissues. /Short chain alkyl-methacrylate esters/
来源:Hazardous Substances Data Bank (HSDB)