(Z)-3-methylhepta-1,5-diene | 50763-51-4
中文名称
——
中文别名
——
英文名称
(Z)-3-methylhepta-1,5-diene
英文别名
3-methyl-1,5-cis-heptadiene;3-Methyl-1,cis-5-heptadien;3-methyl-hepta-1,5c-diene;rac.-cis-3-Methyl-heptadien-(1.5);(5Z)-3-Methyl-1,5-heptadiene;(5Z)-3-methylhepta-1,5-diene
CAS
50763-51-4
化学式
C8H14
mdl
——
分子量
110.199
InChiKey
QIWJMWAOAINRPL-XQRVVYSFSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:112-115 °C
-
密度:0.736±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-甲基-1,5-庚二烯 3-Methyl-1,trans-5-heptadien 50592-72-8 C8H14 110.199 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-甲基-1,5-庚二烯 3-Methyl-1,trans-5-heptadien 50592-72-8 C8H14 110.199
反应信息
-
作为反应物:参考文献:名称:Roth, Wolfgang R.; Staemmler, Volker; Neumann, Martin, Liebigs Annalen, 1995, # 6, p. 1061 - 1118摘要:DOI:
-
作为产物:参考文献:名称:Roth, Wolfgang R.; Staemmler, Volker; Neumann, Martin, Liebigs Annalen, 1995, # 6, p. 1061 - 1118摘要:DOI:
文献信息
-
Evidence for a free radical mechanism in the decomposition of bis(but-2-enyl) tellurium作者:John Stevenson、William Bell、Joseph Ferry、David J. Cole-Hamilton、Janet E. HailsDOI:10.1016/0022-328x(93)80117-t日期:1993.5coupling of the allyl radicals formed, particularly as no compounds containing CH2CHCHMeTe are recovered after partial pyrolysis. The products can be fitted to a purely statistical model in which the reactivity ratio of the primary to secondary allyl is ca. 0.63:0.37. The statistical fit is taken to indicate that mechanisms other than that involving homolytic fission and free radical coupling play a negligibleNa 2 Te的碱性水溶液与MeCH = CHCH 2 Br或CH 2 = MCHMeCl反应,得到ZZ-,ZE-和EE-(MeCH = CHCH 2)2 Te。这是根据在碱性反应条件下涉及Na 2 Te攻击由烯丙基卤形成的2-丁烯基阳离子的机理来解释的。给出E-构型的反应速率为约2。形成Z的3倍。的分解(MeCHCHCH 2)2液相或气相中的Te会产生所有可能的产物,这些产物是由烯丙基的二聚化产生的。这是根据TeC键的均质裂变,然后偶联形成的烯丙基进行解释的,特别是因为部分热解后未回收到含CH 2 = CHCHMeTe的化合物。可以将产物拟合为纯统计模型,其中伯烯丙基与仲烯丙基的反应比为ca。0.63:0.37。统计拟合表明,除涉及均质裂变和自由基偶联的机制外,其他机制所起的作用可忽略不计。
-
Lehmkuhl, Herbert; Fustero, Santos, Liebigs Annalen der Chemie, 1980, # 9, p. 1353 - 1360作者:Lehmkuhl, Herbert、Fustero, SantosDOI:——日期:——
-
Reaction of allylic boron and aluminum "ate" complexes with organic halides and carbonyl compounds. Trialkylboranes as regio-, stereo-, and chemoselective control elements作者:Yoshinori Yamamoto、Hidetaka Yatagai、Kazuhiro MaruyamaDOI:10.1021/ja00398a016日期:1981.4
-
On the regioselectivity of coupling of substituted allyl radicals. Steric versus FMO control作者:Daniel J. Pasto、Gaël L'HermineDOI:10.1016/s0040-4020(01)90155-3日期:1993.4The photo-induced decomposition of substituted-homoallylic 4-nitrobenzenesulfenates produces substituted allyl radicals which undergo dimerization and coupling with the 4-nitrobenzenethiyl radical. The regioselectivity of the dimerization of the allyl radicals is controlled by both steric and FMO properties depending on the nature of the substituent.
-
Salimgareeva, I. M.; Zhebarov, O. Zh.; Bogatova, N. G., Journal of general chemistry of the USSR, 1981, vol. 51, # 2, p. 342 - 346作者:Salimgareeva, I. M.、Zhebarov, O. Zh.、Bogatova, N. G.、Yur'ev, V. P.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
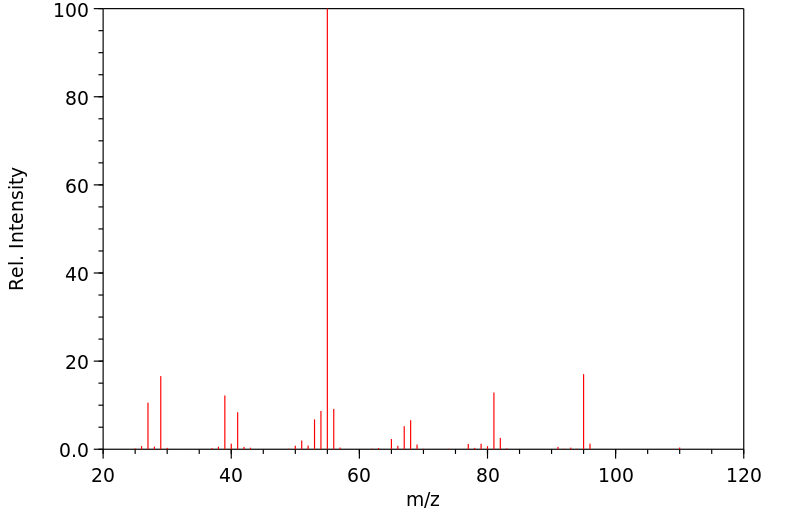
质谱MS
-
碳谱13CNMR
-
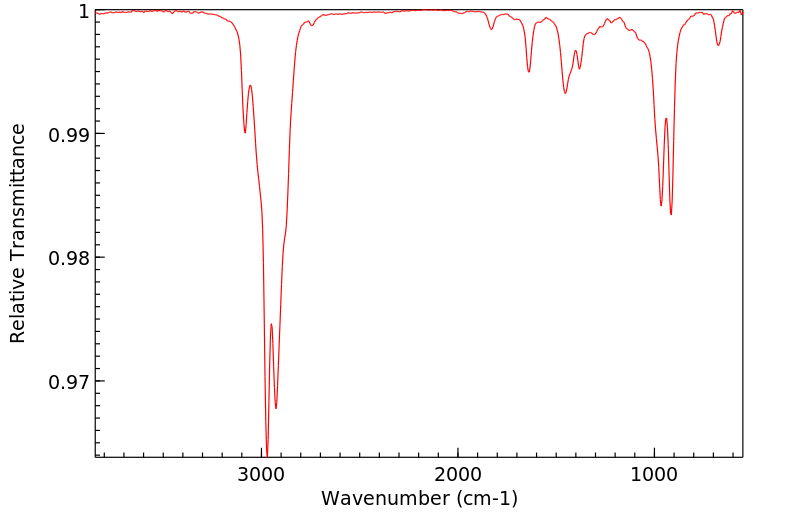
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-