3-乙酰基庚烷-2,6-二酮 | 29214-57-1
中文名称
3-乙酰基庚烷-2,6-二酮
中文别名
——
英文名称
3-acetylheptane-2,6-dione
英文别名
3-acetyl-heptane-2,6-dione;3-acetyl-2,6-heptanedione;2-acetylhepta-2,6-dione;3-acetylhepta-2,6-dione;3-Acetyl-heptan-2,6-dion
CAS
29214-57-1
化学式
C9H14O3
mdl
MFCD00048225
分子量
170.208
InChiKey
RQZJIXZNJBCGQC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:97-101 °C(Press: 0.8 Torr)
-
密度:1.008±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-0.2
-
重原子数:12
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:51.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2914190090
-
储存条件:室温
SDS
反应信息
-
作为反应物:描述:参考文献:名称:无金属的迈克尔加成引发了从1,3-二羰基到多取代吡啶的多组分氧化环脱水路线。摘要:据报道,一种简单的无金属的,经济易行的,可选择性地从易于获得的底物上获得吡啶的方法,涉及一种灵活的4 A分子筛,该分子筛促进了1,3-二羰基,迈克尔受体和环戊烯之间的迈克尔加成引发的多米诺三组分反应。合成当量的氨。DOI:10.1039/b805680c
-
作为产物:参考文献:名称:Photoinitiator, novel compound, and photocurable composition摘要:提供了一种光引发剂,其具有优异的光敏度,产物无色,并可用于厚层紫外线固化涂层。还提供了一种新的化合物,可用于制备光引发剂。同时提供了具有这些特性的光固化组合物。该光引发剂基本上由分子量小于或等于1000的化合物组成,其化学结构由以下公式(1)表示,其中R3和R4独立地表示特定的烷基,R1和R2独立地表示电子吸引基团或特定的烷基,根据化合物的总分子量,以下公式(2)所表示的化学结构元素的重量百分比在17%至54%之间。公开号:US20050256218A1
文献信息
-
Polymer-Incarcerated Gold−Palladium Nanoclusters with Boron on Carbon: A Mild and Efficient Catalyst for the Sequential Aerobic Oxidation−Michael Addition of 1,3-Dicarbonyl Compounds to Allylic Alcohols作者:Woo-Jin Yoo、Hiroyuki Miyamura、Shu̅ KobayashiDOI:10.1021/ja110142y日期:2011.3.9immobilized tetravalent boron catalyst for the Michael reaction. In addition, we found bimetallic Au-Pd nanoclusters to be particularly effective for the aerobic oxidation of allylic alcohols under base- and water-free conditions. The ability to conduct the reaction under relatively neutral and anhydrous conditions proved to be key in maintaining good catalyst activity during recovery and reuse of the catalyst我们开发了一种聚合物包埋的双金属 Au-Pd 纳米团簇和硼作为催化剂,用于 1,3-二羰基化合物与烯丙醇的顺序氧化加成反应。所需的串联反应产物在温和的条件下以良好的收率和广泛的底物范围获得。在我们的研究过程中,我们发现过量的还原剂硼氢化钠与聚合物主链反应生成用于迈克尔反应的固定化四价硼催化剂。此外,我们发现双金属 Au-Pd 纳米团簇在无碱和无水条件下对烯丙醇的有氧氧化特别有效。在相对中性和无水条件下进行反应的能力被证明是在催化剂回收和再利用过程中保持良好催化剂活性的关键。对新制备的 PI/CB-Au/Pd/B 进行结构表征(STEM、EDS、SEM 和 N(2) 吸收/解吸等温线)并与 PI/CB-Au/Pd 进行比较。我们发现,虽然硼对迈克尔加成反应很重要,但发现它会改变聚合物-炭黑复合材料的结构特征,从而对烯丙基氧化反应产生负面影响。
-
Surface-mediated solid phase michael reaction: dramatic acceleration on alumina作者:Brindaban C. Ranu、Sanjay Bhar、Dipak C. SarkarDOI:10.1016/0040-4039(91)85093-k日期:1991.6The Michael reaction of several β-dicarbonyl compounds, ethyl cyanoacetate, and diethyl malonate as donors with methyl vinyl ketone, acrolein and methyl acrylate as acceptors are carried out very efficiently on Al2O3 surface without any solvent.
-
Metal-Free Michael-Addition-Initiated Three-Component Reaction for the Regioselective Synthesis of Highly Functionalized Pyridines: Scope, Mechanistic Investigations and Applications作者:Christophe Allais、Frédéric Liéby-Muller、Jean Rodriguez、Thierry ConstantieuxDOI:10.1002/ejoc.201300246日期:2013.7and completely regioselective three-component synthesis of highly functionalized pyridines from 1,3-dicarbonyl derivatives and Michael acceptors has been achieved. Activated Michael acceptors, that is, β,γ-unsaturated α-oxo carbonyl derivatives, were utilized, allowing substitution at the 4-position and remarkable functional diversity at the 2-position of the pyridine ring. The scope and limitations
-
Cerium(III) Chloride Catalyzed Michael Reaction of 1,3-Dicarbonyl Compounds and Enones in the Presence of Sodium Iodide Under Solvent-Free Conditions作者:Giuseppe Bartoli、Marcella Bosco、Maria Cristina Bellucci、Enrico Marcantoni、Letizia Sambri、Elisabetta TorregianiDOI:10.1002/(sici)1099-0690(199903)1999:3<617::aid-ejoc617>3.0.co;2-r日期:1999.3Cerium(III) chloride heptahydrate in the presence of sodium iodide catalyses the Michael addition of 1,3-dicarbonyl compounds to α,β-unsaturated ketones and α,β-unsaturated aldehydes with extraordinary efficiency. The very mild conditions allow high chemoselectivity as shown by the absence of the typical side reactions, which can be observed in the conventional base-catalyzed processes. More interestingly
-
Ionic Liquid as Catalyst and Reaction Medium. The Dramatic Influence of a Task-Specific Ionic Liquid, [bmIm]OH, in Michael Addition of Active Methylene Compounds to Conjugated Ketones, Carboxylic Esters, and Nitriles作者:Brindaban C. Ranu、Subhash BanerjeeDOI:10.1021/ol051004h日期:2005.7.1[reaction: see text] A task-specific ionic liquid, [bmIm]OH, has been introduced as a catalyst and as a reaction medium in Michael addition. Very interestingly, although the addition to alpha,beta-unsaturated ketones proceeds in the usual way, giving the monoaddition products, this ionic liquid always drives the reaction of open-chain 1,3-dicarbonyl compounds with alpha,beta-unsaturated esters and
表征谱图
-
氢谱1HNMR
-
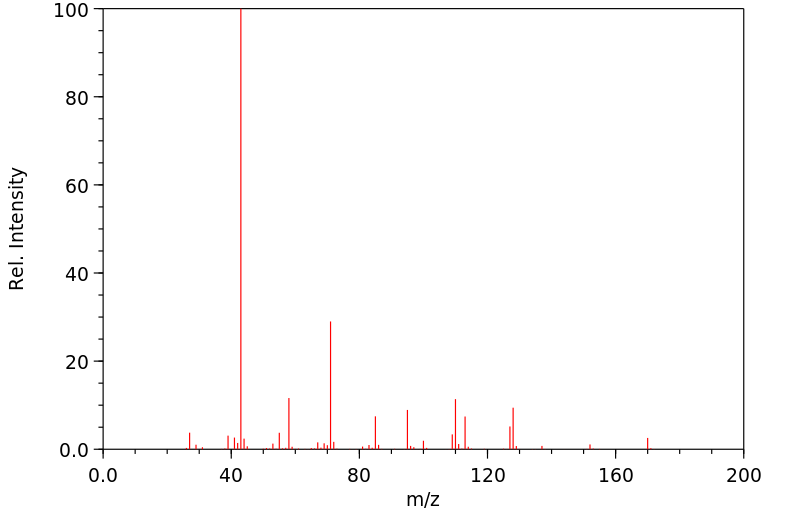
质谱MS
-
碳谱13CNMR
-
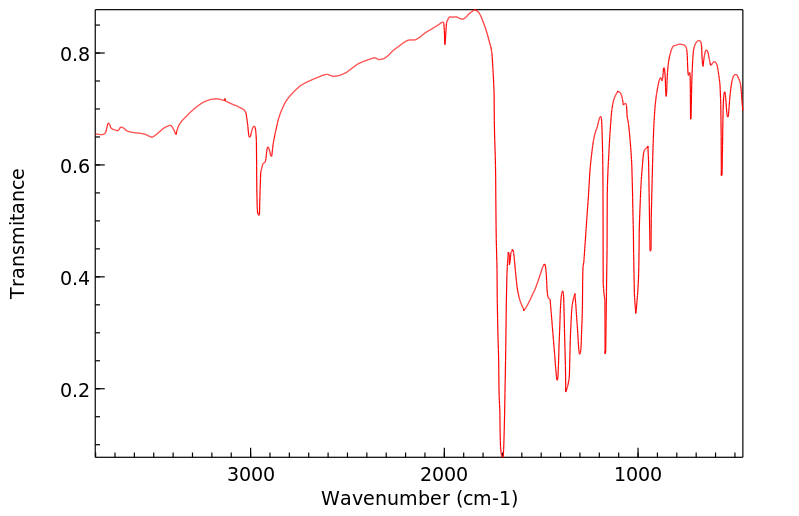
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷