甲磺酸正丁酯 | 1912-32-9
中文名称
甲磺酸正丁酯
中文别名
甲基磺酸丁酯;N-甲烷磺酸丁酯;甲磺酸丁酯;甲烷磺酸丁酯;甲基磺酸正丁酯;甲烷磺酸正丁酯
英文名称
n-butyl methanesulfonate
英文别名
butyl methanesulfonate;butyl mesylate;1-butyl mesylate;n-butyl methanesulphonate
CAS
1912-32-9
化学式
C5H12O3S
mdl
——
分子量
152.214
InChiKey
LFLBHTZRLVHUQC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:172°C (estimate)
-
密度:1.088 (estimate)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:51.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xn
-
安全说明:S23,S36/37
-
危险类别码:R40
-
海关编码:2905199090
-
RTECS号:PB1425000
-
WGK Germany:3
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:库房应保持通风、低温和干燥的环境。
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Peroxides. I. n-Alkyl Hydroperoxides摘要:DOI:10.1021/ja01640a036
-
作为产物:参考文献:名称:Gubaidullin,M.G.; Kovaleva,L.M., Journal of general chemistry of the USSR, 1973, vol. 43, p. 2638 - 2640摘要:DOI:
文献信息
-
Efficient synthesis of organic thioacetates in water作者:F. Olivito、P. Costanzo、M. L. Di Gioia、M. Nardi、Oliverio M.、A. ProcopioDOI:10.1039/c8ob01896k日期:——Thioacetates as precursors of thiols are interesting starting points for synthesizing other organosulfur compounds. Herein, we propose a simple, efficient and fast method to obtain organic thioacetates using water as a solvent. Taking into account the great attention that has been paid toward environmentally friendly synthetic procedures in the past decades, we prove the role and the strength of the
-
Method of preparation of halogen-free ionic liquids and ionic liquids prepared in this manner申请人:Cassol Claudia Cristiana公开号:US20080045723A1公开(公告)日:2008-02-21The reaction of N-alkylimidazol with alkyl sulfonates, at room temperature, favors the production of 1,3-dialkylimidazolium alkane-sulfonates as crystalline solids at high yields. The alkane-sulfonate anions may be easily substituted by a series of other anions [BF 4 , PF 6 , PF 3 (CF 2 CF 3 ) 3 , CF 3 SO 3 and (CF 3 SO 2 ) 2 N] through simple anion, salt, or acid reactions in water at room temperature. The extraction with dichloromethane, filtration, and evaporation of the solvent, allows the production of the desired ionic liquids at a yield of 80-95%. The purity of these ionic liquids (in some cases >99.4%) is performed using the intensity of 13 C satellite signals from the magnetic resonance spectrums of the N-methyl imidazolium group as an internal standard.
-
Synthesis and SAR of New 5-Phenyl-3-ureido-1,5-benzodiazepines as Cholecystokinin-B Receptor Antagonists作者:Antonella Ursini、Anna M. Capelli、Robin A. E. Carr、Paolo Cassarà、Mauro Corsi、Ornella Curcuruto、Giovanni Curotto、Michele Dal Cin、Silvia Davalli、Daniele Donati、Aldo Feriani、Harry Finch、Gabriella Finizia、Giovanni Gaviraghi、Marc Marien、Giorgio Pentassuglia、Stefano Polinelli、Emiliangelo Ratti、Aldo Reggiani、Giorgio Tarzia、Giovanna Tedesco、Maria E. Tranquillini、David G. Trist、Frank T. M. Van AmsterdamDOI:10.1021/jm990967h日期:2000.10.1A series of 5-phenyl-3-ureidobenzodiazepine-2,4-diones was synthesized and evaluated as cholecystokinin-B (CCK-B) receptor antagonists. Structure-activity relationship (SAR) studies revealed the importance of the N-1 substituent for potent and selective CCK-B affinity. Addition of substituents at the urea side chain provided in some cases more potent compounds. Moreover the introduction of bulky substituents
-
Rhodium-Catalyzed Carbonylative Coupling of Alkyl Halides with Phenols under Low CO Pressure作者:Han-Jun Ai、Hai Wang、Chong-Liang Li、Xiao-Feng WuDOI:10.1021/acscatal.0c00933日期:2020.5.1A rhodium-catalyzed carbonylative transformation of alkyl halides under low pressure of CO has been developed. This robust catalyst system allows using phenols as the carbonylative coupling partner and, meanwhile, exhibits high functional group tolerance and good chemoselectivity. Substrates even with a large steric hindrance group or multiple reaction sites can be selectively converted into the desired
-
[EN] NOVEL PIPERAZINYL-PYRAZINONE DERIVATIVES FOR THE TREATMENT OF 5-HT2A RECEPTOR-RELATED DISORDERS<br/>[FR] NOUVEAUX DERIVES DE PIPERAZINYL-PYRAZINONE POUR LE TRAITEMENT DES TROUBLES LIES AU RECEPTEUR 5-HT2A申请人:BIOVITRUM AB公开号:WO2004009586A1公开(公告)日:2004-01-29Compounds of the general formula (I): (I)wherein m, n, R1, R2, R3 and R4 are as described in the specification. Further included are pharmaceutical compositions comprising the compounds, processes for their preparation, as well as the use of the compounds for the preparation of a medicament for the treatment of 5-HT2A receptor-related disorders or medical conditions.通式(I)的化合物:(I)其中m、n、R1、R2、R3和R4如规范中所述。还包括包含这些化合物的药物组合物,它们的制备方法,以及利用这些化合物制备用于治疗5-HT2A受体相关疾病或医疗状况的药物的用途。
表征谱图
-
氢谱1HNMR
-
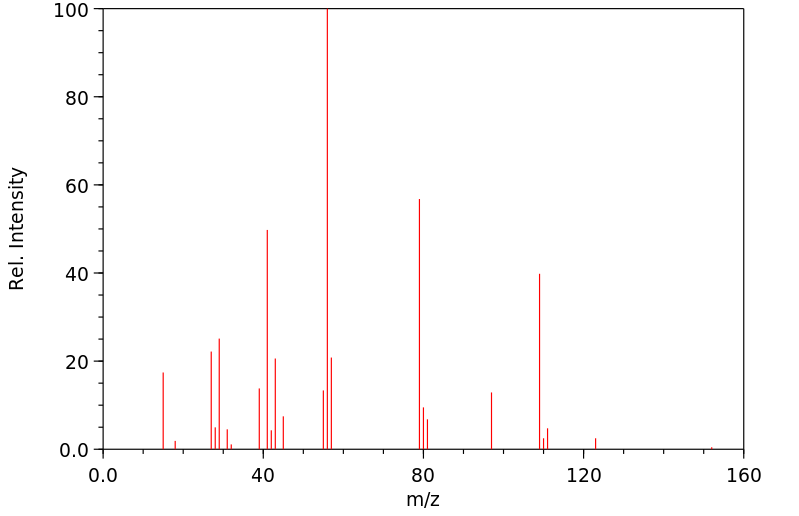
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高烯丙基(甲磺酰基)胺
高炔丙基(甲磺酰基)胺
顺式-9-十八碳烯基甲烷磺酸酯
顺式-4-乙基环己基甲烷磺酸酯
顺-1,2-双(甲磺酰基氧基甲基)环己烷
阿坎酸杂质
阿坎酸
锌甲烷磺酸盐
铵磺酸甜菜碱-3
铵磺酸甜菜碱-2
铵磺酸甜菜碱-1
铬雾抑制剂
铁三(三氟甲基磺酰基)亚胺
钾3-(三羟基硅烷基)-1-丙烷磺酸酯
钾1,1,2,2,3,3,4,4-八氟丁烷-1-磺酸盐
钡二乙烷磺酸酯
钠3-氨基丙烷磺酸酯
钠3-氨基-3-氧代-丙烷-1-磺酸酯
钠3-(三羟基硅烷基)-1-丙烷磺酸酯
钠2-[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-十八碳-9-烯氧基乙氧基)乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]-2-氧代-乙烷磺酸酯
钠1-羟基-1-庚烷磺酸酯
钠(1E)-1-十二碳烯-1-磺酸酯
酪朊酸钠
辛烷-1-磺酸甲酯
辛烷-1-磺酸乙酯
辛基-1-磺酸戊酯
辛-2-烯-1-磺酸
辅酶 M
西尼必利杂质7
萘-1,8-二甲醇
英丙舒凡对甲苯磺酸盐
英丙舒凡
苯基硒基三氟甲烷磺酸酯
芥酸酰胺丙基羟基磺基甜菜碱
艾日布林中间体
脒基牛磺酸
胺磺酸甜菜碱-4
联硫亚盐氯乙醛钠水合物
羧基-五聚乙二醇-磺酸
羟甲基磺酸钠
羟基甲烷磺酸铵盐
羟基甲烷磺酸钾
羟基甲氧基甲醇甲磺酸酯
羟乙磺酸钾
羟乙基磺酸铵盐
羟乙基磺酸钠
羟丙基硫代硫酸钠
美司那
磺酸钠
磺酸己烷