甲酸乙烯酯 | 692-45-5
中文名称
甲酸乙烯酯
中文别名
——
英文名称
vinyl formate
英文别名
formic acid ethenyl ester;methyloyloxyethylene;ethenyl formate
CAS
692-45-5
化学式
C3H4O2
mdl
MFCD00046142
分子量
72.0636
InChiKey
GFJVXXWOPWLRNU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:16.85°C
-
沸点:46-47°C
-
密度:0,959 g/cm3
-
闪点:16°C
-
蒸汽压力:312.97 mmHg
-
保留指数:1090
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:5
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
危险类别码:R11
-
危险品运输编号:UN 3272
-
海关编码:2915120000
-
包装等级:O53
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙酸乙烯酯 vinyl acetate 108-05-4 C4H6O2 86.0904
反应信息
-
作为反应物:描述:参考文献:名称:Radical reactions of tetrafluorohydrazine. Preparation of bis(difluoramino)alkanols and nitrates摘要:DOI:10.1021/jo00986a027
-
作为产物:描述:参考文献:名称:DE637257摘要:公开号:
-
作为试剂:描述:参考文献:名称:Abramow; Zyplenkowa, Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1944, p. 60,62摘要:DOI:
文献信息
-
Reactions of dichlorocarbene and tri-chloromethide with O-alkenyl esters and ethers, N-vinyl amides, and 1-haloalkenes作者:R.C. De Selms、T.-W. LinDOI:10.1016/0040-4020(67)85101-9日期:1967.1mixtures due to apparent addition of trichloromethide and dichlorocarbene. In general, the yields of gem-dichlorocyclopropanes increased with increasing alkyl substituents on the alkene substrates. A trend toward increasing yield of gem-dichlorocyclopropanes was also noticed with the following substituents on the alkene substrates: OP(O) (OR)2 < Halogen ≅ OC(O)R < OR ≅ NRC(O)R′. Evidence is presented据报道,由三氯乙酸钠产生的二氯卡宾和/或三氯甲烷在回流的1,2-二甲氧基乙烷中与各种脂族O-炔烃酯和醚,N-乙烯基酰胺和1-卤代烯烃反应的范围和局限性。所有组均给出了宝石-二氯环丙烷,唯一的例外是O-乙烯基羧酸酯,其中仅出现了三氯甲基化物的加成产物或由于明显添加了三氯甲基化物和二氯卡宾而形成的混合物。通常,宝石-二氯环丙烷的产率随着烯烃底物上烷基取代基的增加而增加。还发现在烯烃底物上带有以下取代基的宝石-二氯环丙烷的收率有增加的趋势:OP(O)(OR)2 <卤素OC(O)R <或ORNRC(O)R'。提供的证据表明质子的来源与三氯甲基甲烷的添加同时发生。详细介绍了一些取代的宝石-二氯环丙烷与LAH的反应。
-
[EN] PROCESS INCLUDING HYDROGENOLYSIS OF BIOMASS FOLLOWED BY DEHYDROGENATION AND ALDOL CONDENSATION FOR PRODUCING ALKANES<br/>[FR] PROCÉDÉ INCLUANT L'HYDROGÉNOLYSE D'UNE BIOMASSE SUIVIE D'UNE DÉSHYDROGÉNATION ET D'UNE CONDENSATION ALDOLIQUE POUR LA PRODUCTION D'ALCANES申请人:SHELL OIL CO公开号:WO2011143392A1公开(公告)日:2011-11-17A method comprises providing a bio-based feedstock; contacting the bio-based feedstock with a solvent in a hydrolysis reaction to form an intermediate stream comprising carbohydrates; contacting the intermediate stream with an aqueous phase reforming catalyst to form a plurality of oxygenated intermediates, wherein a first portion of the oxygenated intermediates are recycled to form the solvent; and contacting at least a second portion of the oxygenated intermediates with a condensation catalyst comprising a base functionality to form a fuel blend.
-
Regioselective lipase-catalysed acylation of 4,6-O-benzylidene-α- and-β-d-pyranoside derivatives displaying a range of anomeric substituents作者:Jonathan J. Gridley、Andrew J. Hacking、Helen M.I. Osborn、David G. SpackmanDOI:10.1016/s0040-4020(98)00935-1日期:1998.12The application of Lipase enzymes to effect regioselective C-3-O-acylation of - and -galactopyranosides displaying a range of anomeric substituents, and C-2-O-acylation of phenyl and ethyl is reported. In particular this method has allowed introduction of a variety of acyl protecting groups at the C-3 hydroxyl group of ethyl 11.脂肪酶的到应用的效果的区域选择性C-3- Ö的酰化-和-galactopyranosides显示范围的端基异构体的取代基,和C-2- Ö苯基的酰化和乙基报道。特别地,该方法允许在乙基11的C-3羟基处引入各种酰基保护基。
-
Unusual stereoselectivity in Diels-Alder cycloadditions of 5-bromopyrone作者:Kamyar Afarinkia、Natasha T. Daly、Silvia Gomez-Farnos、Shravan JoshiDOI:10.1016/s0040-4039(97)00352-3日期:1997.35-Bromopyrone undergoes cycloaddition to poorly activated or unactivated alkenes to afford high yields of cycloadducts. The regiochemistry of the cycloaddition is excellent. The stereoselectivity of cycloaddition depends on both electronic and steric factors in the dienophile but can be controlled to give predominantly endo or predominantly exo cycloadducts.
-
Reaktionen von aluminiumalkylen mit carbonyl-verbindungen作者:Siegfried Warwel、Günter Schmitt、Friedrich AsingerDOI:10.1016/s0022-328x(00)80671-3日期:1972.3Under moderate conditions aldoenol esters react with HAl(i-C4H9)2 to afford organoaluminium compounds, which upon hydrolysis yield 1,3-diols and on acetolysis form 1,3-diacetates. The diacetates are obtained in about 50% yields (based on enol ester) by distillation. Vinyl esters are converted to linear 1,3-diols or 1,3-diacetates, whereas enol acetates of higher aldehydes give 2-sec(or -tert)alkyl-1
表征谱图
-
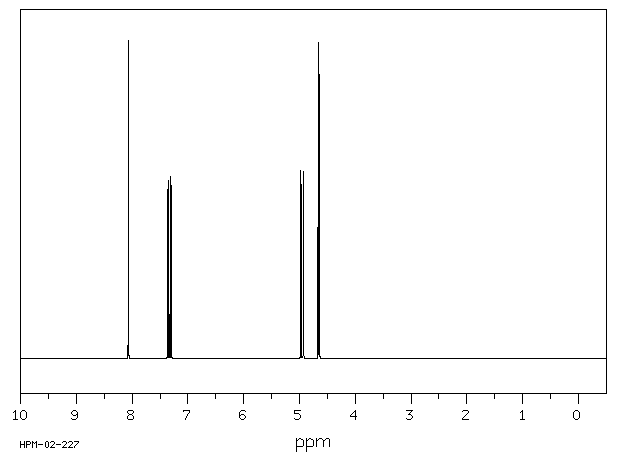
氢谱1HNMR
-
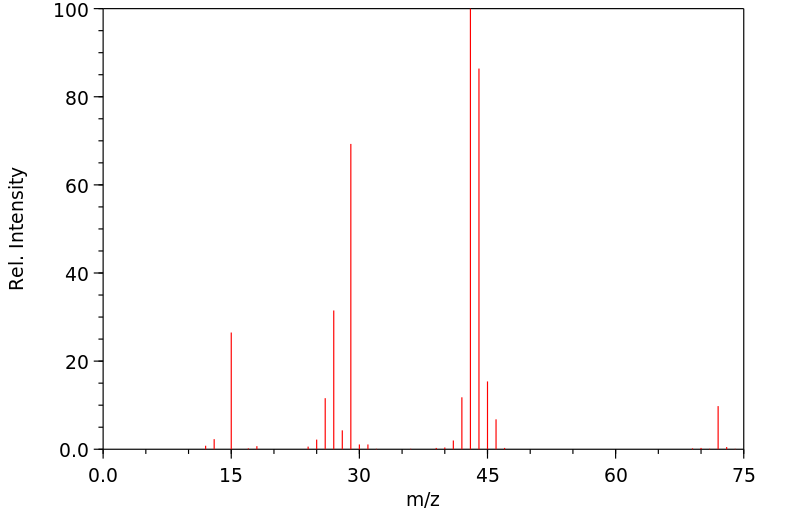
质谱MS
-
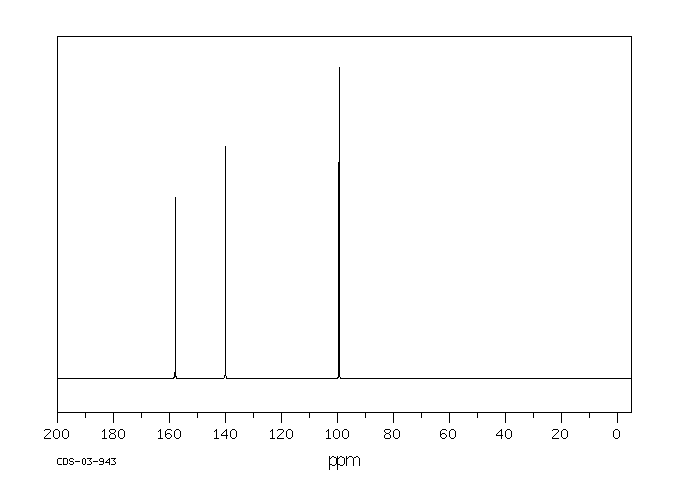
碳谱13CNMR
-
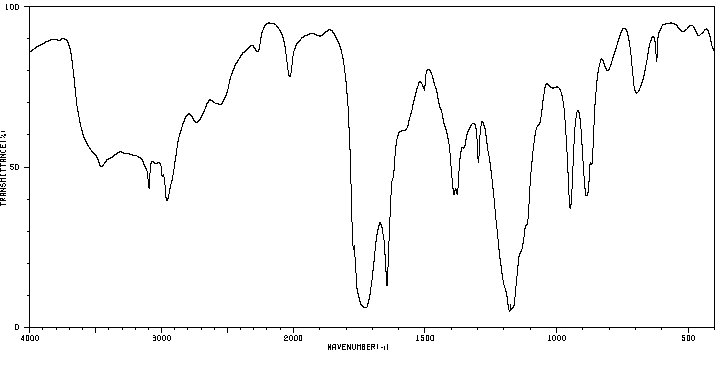
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸