盐酸妥拉唑林 | 59-97-2
中文名称
盐酸妥拉唑林
中文别名
苯甲唑啉盐酸盐;2-苄基咪唑啉;2-苄基-2-咪唑啉盐酸盐;盐酸苯甲唑林;苄唑啉;苯甲唑啉;妥拉苏林;2-苄基咪唑啉盐酸盐
英文名称
tolazoline hydrochloride
英文别名
2-benzyl-2-imidazoline hydrochloride;2-benzyl-4,5-dihydro-1H-imidazole;hydron;chloride
CAS
59-97-2
化学式
C10H12N2*ClH
mdl
MFCD00012693
分子量
196.68
InChiKey
RHTNTTODYGNRSP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:172-176 °C
-
溶解度:DMSO(微溶,加热)、甲醇(微溶)
-
碰撞截面:135.1 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
稳定性/保质期:
Hygroscopic
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:13
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:24.4
-
氢给体数:2
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S22
-
危险类别码:R22
-
海关编码:2933290090
-
WGK Germany:3
-
危险品运输编号:2811
-
储存条件:本品应密封保存。
SDS
| Name: | Tolazoline Hydrochloride 99% Material Safety Data Sheet |
| Synonym: | 2-Benzyl-2-Imidazoline Hydrochloride |
| CAS: | 59-97-2 |
Synonym:2-Benzyl-2-Imidazoline Hydrochloride
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 59-97-2 | Tolazoline Hydrochloride | 99% | 200-447-3 |
Risk Phrases: 22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 59-97-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: white
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 174.00 - 176.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: freely soluble
Specific Gravity/Density:
Molecular Formula: C10H12N2.HCl
Molecular Weight: 196.72
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 59-97-2: NJ2350000 LD50/LC50:
CAS# 59-97-2: Oral, mouse: LD50 = 400 mg/kg; Oral, rat: LD50 = 1200 mg/kg.
Carcinogenicity:
Tolazoline Hydrochloride - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 22 Harmful if swallowed.
Safety Phrases:
S 22 Do not breathe dust.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 59-97-2: No information available.
Canada
CAS# 59-97-2 is listed on Canada's NDSL List.
CAS# 59-97-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 59-97-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
生物活性
体外研究
Tolazoline HCl 是一种非选择性和竞争性的 α-肾上腺素能受体拮抗剂。
靶点| Target | Value |
|---|---|
| α-adrenergic receptor |
Tolazoline 是一种肺血管扩张药,在持续肺动脉高压症的新生儿体内(PPHN)用于减弱肺血管阻力 (PVR)。它具有适度的α-肾上腺素能阻滞活性和组胺激动剂活性,通常用于降低肺动脉压和血管阻力。在所有调查的致痉挛药物中,Tolazoline 没有表现出广泛的SNP作用范围。然而,在人桡动脉中,它可能能够有效抵消 α-肾上腺素能受体介导的血管痉挛。
用途- 作为血管扩张药
- 用于 α-adrenergic 受体拮抗剂和外周血管扩张剂
有毒物品
毒性分级高毒
急性毒性口服 - 大 LD50: 1200 毫克/公斤;口服 - 小鼠 LD50: 400 毫克/公斤
可燃性危险特性燃烧产生有毒氮氧化物和氯化氢烟雾,同时具有药物副作用,包括心跳加速、出汗、十二指肠出血和溃疡。
储运特性通风、低温干燥
灭火剂反应信息
-
作为反应物:描述:参考文献:名称:重氮盐离子级联反应,来自于含咪唑啉的药物托拉唑啉的亚硝化。摘要:托拉唑啉(1-苄基咪唑啉)是一种代表性的含咪唑啉的药物,在乙酸中容易与亚硝酸盐反应生成复杂的产物混合物。当使用8倍过量的HNO2时,已鉴定出14种化合物是该转化的产物。包括N-亚硝基酰胺,酯,醇和苯乙酸在内的产品被合理化为由一系列反应性重氮离子产生。当随着转化的进行用CH 2 Cl 2萃取混合物时,可以以高收率从亚硝化反应中分离出N-亚硝基偶氮唑啉。它比托拉唑啉的亚硝化速度快得多(50x),从而得到肟[1-(N-亚硝基-2-咪唑啉基)亚苄基]羟胺,该化合物也可以通过托拉唑啉与异丙基的反应以高收率生产。亚硝酸盐。在低底物和亚硝酸盐浓度下,主要反应产物为N-亚硝基偶氮唑啉,其分解产物N-2-羟乙基苯基乙酰胺,上述肟,苯基乙酸和2-羟乙基苯基乙酸酯。在pH 3.4和37摄氏度下测定了三种缓冲液系统中托拉唑啉的亚硝化率(在0.5 M乙酸盐缓冲液中使用10 * [NO2(-)] = 250时,kobsDOI:10.1021/tx700317g
文献信息
-
1-(2-PHENOXYMETHYLHETEROARYL)PIPERIDINE AND PIPERAZINE COMPOUNDS申请人:Stangeland Eric L.公开号:US20110230495A1公开(公告)日:2011-09-22The invention relates to compounds of formula I: where X, HAr, a, and R 1 through R 6 are as defined in the specification, or a pharmaceutically acceptable salt thereof. The compounds of formula I are serotonin and norepinephrine reuptake inhibitors. The invention also relates to pharmaceutical compositions comprising such compounds; methods of using such compounds; and process and intermediates for preparing such compounds.
-
4-[2-(2-FLUOROPHENOXYMETHYL)PHENYL]PIPERIDINE COMPOUNDS申请人:Patterson Lori Jean公开号:US20100125092A1公开(公告)日:2010-05-20The invention relates to compounds of formula I: where a, R 1 , and R 3-6 are as defined in the specification, or a pharmaceutically acceptable salt thereof. The compounds of formula I are serotonin and norepinephrine reuptake inhibitors. The invention also relates to pharmaceutical compositions comprising such compounds; methods of using such compounds; and process and intermediates for preparing such compounds.
-
ARYLOAZOL-2-YL CYANOETHYLAMINO COMPOUNDS, METHOD OF MAKING AND METHOD OF USING THEREOF申请人:Soll Mark David公开号:US20140080862A1公开(公告)日:2014-03-20The present invention relates to novel aryloazol-2-yl-cyanoethylamino derivatives of formula (I): wherein R 3 , R 4 , R 5 , R 6 , R 7 , P, Q, V, W, X, Y, Z and a are as defined in the description, compositions thereof, processes for their preparation and their uses as pesticides.
-
[EN] PRODRUG COMPOSITIONS AND METHODS OF TREATMENT<br/>[FR] COMPOSITIONS DE PROMÉDICAMENT ET PROCÉDÉS DE TRAITEMENT申请人:AQUESTIVE THERAPEUTICS INC公开号:WO2021087359A1公开(公告)日:2021-05-06Pharmaceutical compositions include a prodrug of epinephrine are described.药物组合物包括表述的肾上腺素前药。
-
Multi-functional ionic liquid compositions for overcoming polymorphism and imparting improved properties for active pharmaceutical, biological, nutritional, and energetic ingredients申请人:Rogers D. Robin公开号:US20070093462A1公开(公告)日:2007-04-26Disclosed are ionic liquids and methods of preparing ionic liquid compositions of active pharmaceutical, biological, nutritional, and energetic ingredients. Also disclosed are methods of using the compositions described herein to overcome polymorphism, overcome solubility and delivery problems, to control release rates, add functionality, enhance efficacy (synergy), and improve ease of use and manufacture.
表征谱图
-
氢谱1HNMR
-
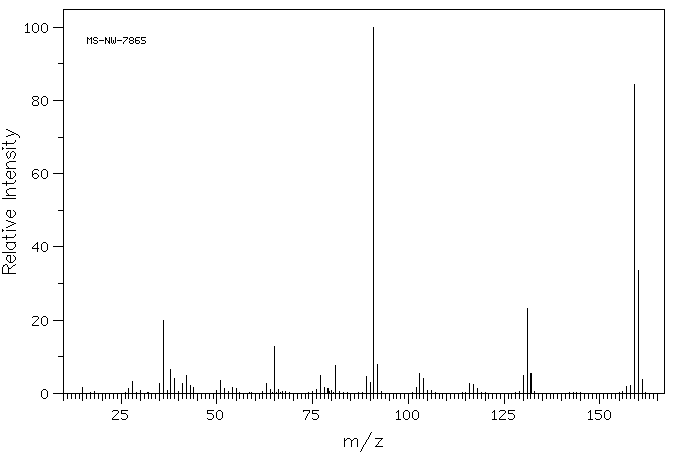
质谱MS
-
碳谱13CNMR
-
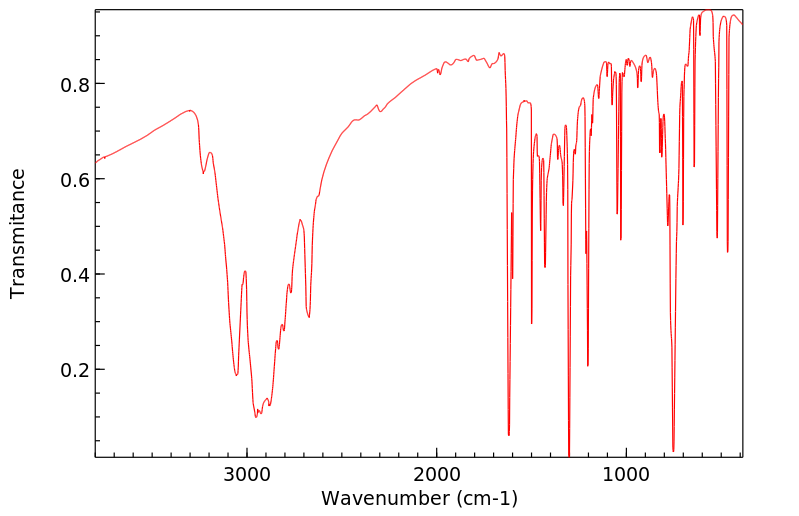
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫