4-巯基-4-甲基-2-戊醇 | 31539-84-1
中文名称
4-巯基-4-甲基-2-戊醇
中文别名
——
英文名称
4-mercapto-4-methylpentan-2-ol
英文别名
4-Mercapto-4-methyl-2-pentanol;4-methyl-4-sulfanylpentan-2-ol
CAS
31539-84-1
化学式
C6H14OS
mdl
MFCD10688294
分子量
134.243
InChiKey
FDBQLLMYSACLPB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:40℃/0.6mm
-
密度:0.957
-
闪点:70°(158°F)
-
LogP:1.28
-
物理描述:Clear, colourless to pale yellow liquid; Floral, fruity aroma
-
溶解度:Soluble in water
-
折光率:1.463-1.468
-
保留指数:1042;952;952;947
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:21.2
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2930909090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-mercapto-4-methylpent-2-yl formate 50746-12-8 C7H14O2S 162.253
反应信息
-
作为反应物:描述:4-巯基-4-甲基-2-戊醇 在 正丁基锂 、 硫酸 作用下, 以 水 为溶剂, 生成 (2R)-2-[(2R,6R)-4,4,6-trimethyl-1,3-oxathian-2-yl]butan-2-ol参考文献:名称:基于 1,3-氧杂环己烷的不对称合成。一、反应范围摘要:非对映选择性反应练习曲 d'acyl ou aroyl-2 trimethyl-4,4,6- ou -4,6,6 oxathiannes-1,3 avec des OrganicMetalliques (RMgX, R 2 Mg ou RLi) en fonction des variables suvantes : 取代 (4,6,6 或 4,4,6),nature du groupe acyl-2,nature de l'organometallique et de l'halogene dans le cas de RMgX,溶剂,温度;非对映异构体的最佳条件DOI:10.1021/ja00322a033
-
作为产物:描述:参考文献:名称:偏高碘酸钠氧化3-硫烷基醇:苏丹嘌呤的新合成摘要:描述了一种简单高效的方法,该方法通过用偏高碘酸钠氧化3-硫烷基醇来制备磺胺类药物。DOI:10.1016/s0040-4039(97)10757-2
文献信息
-
Preparation and Assessment of Self-Immolative Linkers for Therapeutic Bioconjugates with Amino- and Hydroxyl-Containing Cargoes作者:Myagmarsuren Sengee、J. Johannes Eksteen、Silje Lillemark Nergård、Terje Vasskog、Leiv K. SydnesDOI:10.1021/acs.bioconjchem.9b00214日期:2019.5.15A series of self-immolative linkers containing a thiol-reactive group at one end and a hydroxyl- or amine-reactive group at the other were prepared. The utility of these reagents for preparations of bioconjugates was explored by reacting the linkers with appropriately functionalized model drugs and peptides. Degradation studies of a series of conjugates with different linkers reveal that the structure
-
Mercapto alcohols and mercaptoalkyl esters申请人:Polak's Frutal Works B.V.公开号:US03970689A1公开(公告)日:1976-07-20New compounds having the formula ##EQU1## wherein R.sub.1 represents a hydrocarbon moiety having from 1 to 7 carbon toms, R.sub.2 is hydrogen, methyl or ethyl, and R.sub.3 is hydrogen, formyl or acetyl. The compounds are useful in the preparation, modification and intensification of a great variety of flavor and perfume compositions.
-
PRECURSOR COMPOUNDS OF SWEET TASTE RECEPTOR ANTAGONISTS FOR THE PREVENTION OR TREATMENT OF DISEASE申请人:Ley Jakob Peter公开号:US20110045069A1公开(公告)日:2011-02-24A description is given of precursor compounds of sweet taste receptor antagonists for the prevention or treatment of disease, in particular for the prevention or treatment of Type 2 diabetes. A description is also given of uses of these precursor compounds and edible compositions, preparations for nutrition or pleasure or semi-finished products and pharmaceutical preparations, containing such precursor compounds.描述了甜味受体拮抗剂的前体化合物,用于预防或治疗疾病,特别是用于预防或治疗2型糖尿病。还描述了这些前体化合物的用途,以及包含这些前体化合物的食用组合物、营养或愉悦制剂、半成品和含有这些前体化合物的药物制剂。
-
SULFIDE-BASED COMPOUNDS AND USES THEREOF申请人:Ecolab USA Inc.公开号:US20200109112A1公开(公告)日:2020-04-09Disclosed are sulfide-based compounds which are a product of a Michael addition reaction between a sulfur-containing donor group and an unsaturated hydrocarbon moiety. The sulfide-based compounds may be used in compositions and methods for inhibiting corrosion.
-
Use of ure2 mutant yeasts for increasing the release of aromatic volatile thiols by yeast during fermentation
表征谱图
-
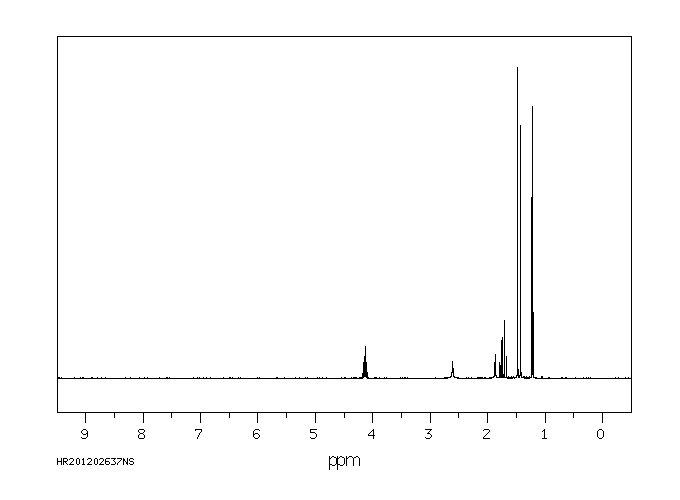
氢谱1HNMR
-
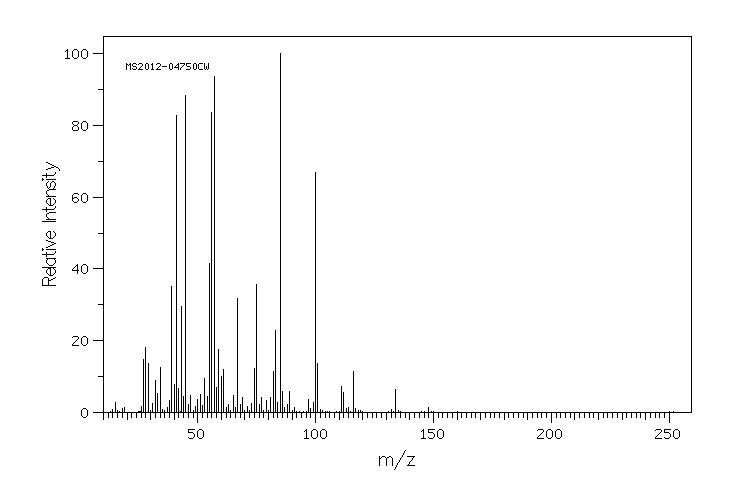
质谱MS
-
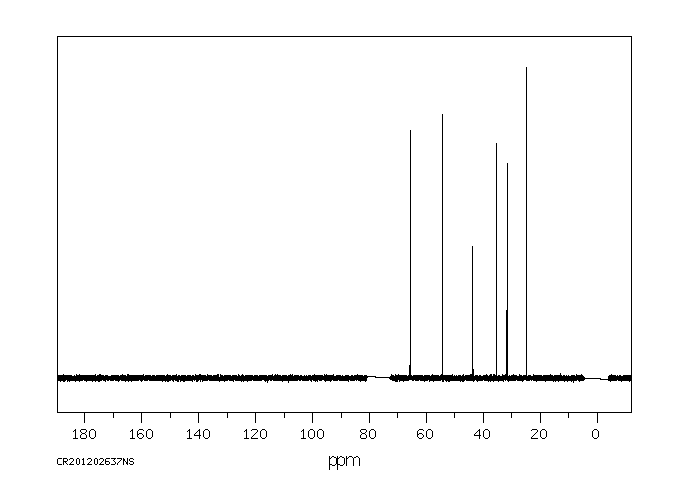
碳谱13CNMR
-
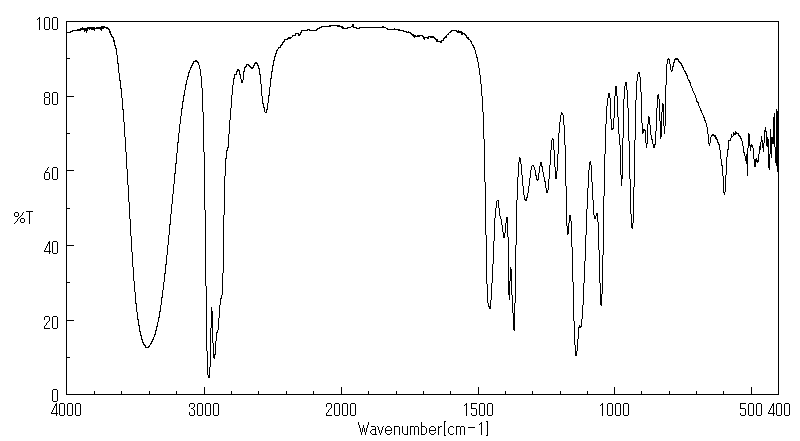
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷