章鱼碱 | 34522-32-2
中文名称
章鱼碱
中文别名
(+)-真蛸碱;章魚肉鹼
英文名称
N2-(D-1-carboxyethyl)-L-arginine
英文别名
(R),(S)-octopine;octopine;Nα-((R)-1-carboxy-ethyl)-L-arginine;Nα-((R)-1-Carboxy-aethyl)-L-arginin;D-Octopin;L-Arginine, N2-[(1R)-1-carboxyethyl]-;(2S)-2-[[(1R)-1-carboxylatoethyl]azaniumyl]-5-(diaminomethylideneazaniumyl)pentanoate
CAS
34522-32-2
化学式
C9H18N4O4
mdl
——
分子量
246.266
InChiKey
IMXSCCDUAFEIOE-RITPCOANSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:283-284 °C(lit.)
-
沸点:389.26°C (rough estimate)
-
密度:1.2480 (rough estimate)
-
溶解度:甲醇(微溶)、水(微溶、加热、超声处理)
计算性质
-
辛醇/水分配系数(LogP):-3.7
-
重原子数:17
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:151
-
氢给体数:5
-
氢受体数:6
安全信息
-
安全说明:S24/25
-
海关编码:2925290090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (+)-八蒎酸 (R),(S)-octopinic acid 20197-09-5 C8H16N2O4 204.226 L-精氨酸 L-arginine 74-79-3 C6H14N4O2 174.203 DL-精氨酸 Arg 7200-25-1 C6H14N4O2 174.203 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (+)-八蒎酸 (R),(S)-octopinic acid 20197-09-5 C8H16N2O4 204.226 —— 5-Amino-2-[(1-carboxyethyl)amino]pentanoic acid —— C8H16N2O4 204.22
反应信息
-
作为反应物:参考文献:名称:摘要:DOI:
-
作为产物:描述:(R)-2-bromo-5-acetamidopentanoic acid 在 盐酸 、 barium dihydroxide 、 sodium hydroxide 作用下, 反应 154.0h, 生成 章鱼碱参考文献:名称:天然章鱼中丙氨酸部分的构型摘要:章鱼碱具有N2-(1-羧乙基)精氨酸或N-(1-羧基-4-胍基丁基)丙氨酸的结构。天然章鱼碱中精氨酸部分的构型被确定为(S),而丙氨酸部分的构型被假定为(R)。通过从 (R)-丙氨酸和 (R)-2 开始的一系列反应,通过 (+)-章鱼碱的当前合成得出丙氨酸部分的构型为 (R) -bromo-5-acetamidopentanoic 酸。(R)-丙氨酸和(R)-2-溴-5-乙酰氨基戊酸在碱性条件下反应得到N2-((R)-1-羧乙基)-N5-乙酰基-(S)-鸟氨酸,其转化为N2-((R)-1-羧乙基)-(S)-鸟氨酸((R),(S)-章鱼酸)通过用盐酸水解。(R),(S)-章鱼碱酸通过胍化作用得到(+)-章鱼碱((R),(S)-章鱼碱)。DOI:10.1246/bcsj.55.261
文献信息
-
ACE-2 modulating compounds and methods of use thereof申请人:——公开号:US20040082496A1公开(公告)日:2004-04-29ACE-2 modulating compounds for the treatment of body weight disorders are disclosed. Methods of using the compounds and pharmaceutical compositions containing the compounds are also claimed.抗ACE-2调节化合物用于治疗体重失调的方法已被披露。还声称了使用这些化合物的方法和含有这些化合物的药物组合物。
-
Synthesis and isolation of octopine and its analogues申请人:The University of Alabama in Birmingham公开号:US04615976A1公开(公告)日:1986-10-07Novel octopine derivatives are disclosed which are useful for the selective growth of the agriculturally useful A. tumefaciens bacterium along with a synthetic method for their preparation. Also disclosed is a method of separating octopine derivatives from their diastereoisomers along with a method of selectively growing strains of the bacterium A. tumefaciens.
-
Putative reaction mechanism of heterologously expressed octopine dehydrogenase from the great scallop, Pecten maximus (L)作者:Andre Müller、Frank Janßen、Manfred K. GrieshaberDOI:10.1111/j.1742-4658.2007.06151.x日期:2007.12scallop's adductor muscle. Site-directed mutagenesis was used to elucidate the involvement of several amino acid residues for the reaction catalyzed by ODH. Cys148, which is conserved in all opine dehydrogenases known to date, was converted to serine or alanine, showing that this residue is not intrinsically important for catalysis. His212, Arg324 and Asp329, which are also conserved in all known opine dehydrogenase使用5'-和3'-RACE克隆了大扇贝内收肌的章鱼碱脱氢酶(ODH)的cDNA。cDNA包含1197个核苷酸的ORF,推导的氨基酸序列编码399个氨基酸的蛋白质。ODH在大肠杆菌中异源表达,带有一个C端五角组His-tag。使用金属螯合亲和色谱和Sephadex G-100凝胶过滤将ODH-5His纯化至均质。重组ODH的动力学性质与从扇贝内收肌分离的野生型ODH相似。使用定点诱变来阐明ODH催化的反应中涉及多个氨基酸残基。迄今为止已知的所有阿片脱氢酶中都保守的Cys148已转化为丝氨酸或丙氨酸,表明该残留物对于催化并不是本质上重要的。在所有已知的阿片脱氢酶序列中也都保守的His212,Arg324和Asp329进行了定点诱变。这些残基的修饰揭示了它们对酶催化活性的重要性。这些残基向丙氨酸的转化导致丙酮酸和L-精氨酸的K(m)大大增加而k(cat)值降低,但对NADH的K(m)和k(cat)值影响很小。
-
Herbst; Swart, Journal of Organic Chemistry, 1946, vol. 11, p. 375作者:Herbst、SwartDOI:——日期:——
-
Akasi, Journal of Biochemistry, 1937, vol. 25, p. 283作者:AkasiDOI:——日期:——
表征谱图
-
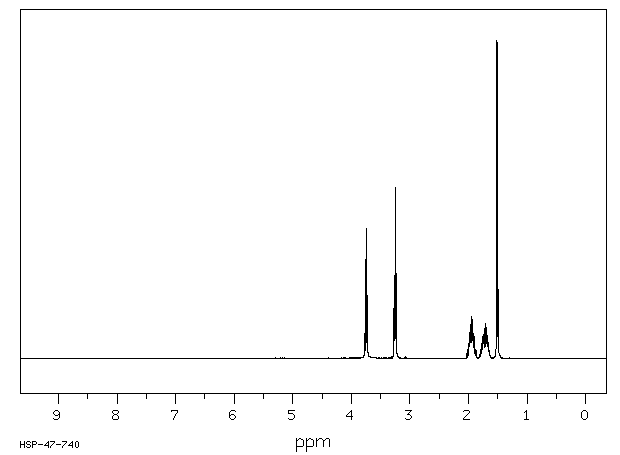
氢谱1HNMR
-
质谱MS
-
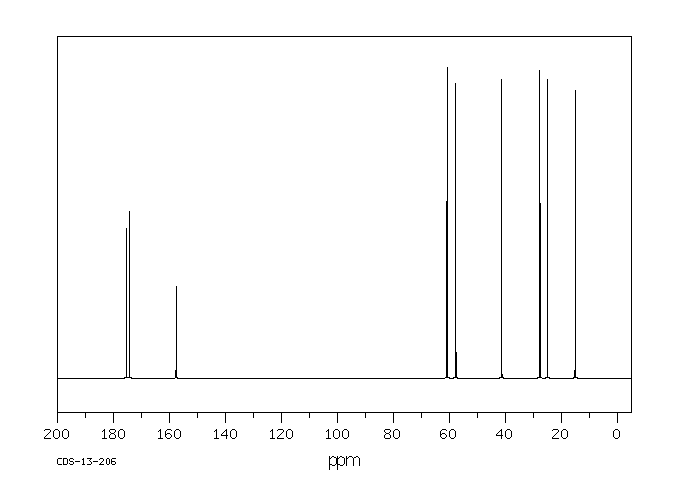
碳谱13CNMR
-
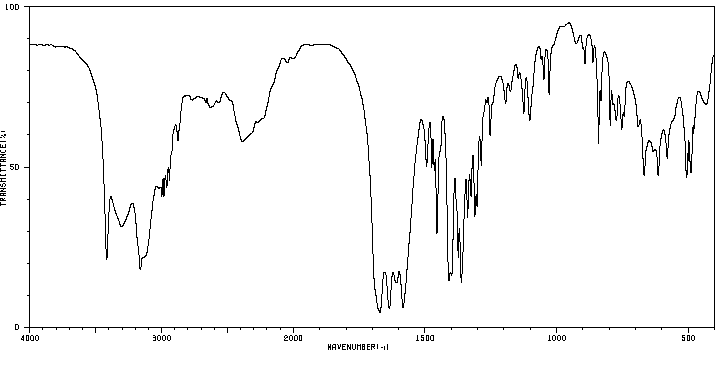
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸