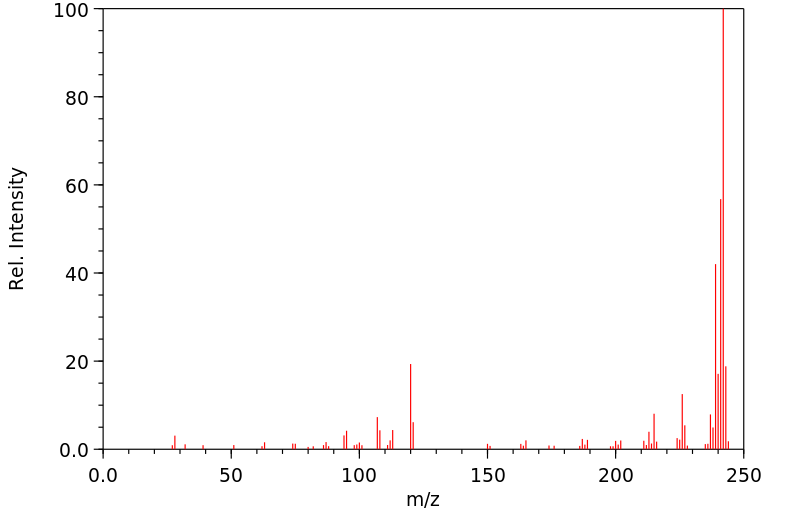
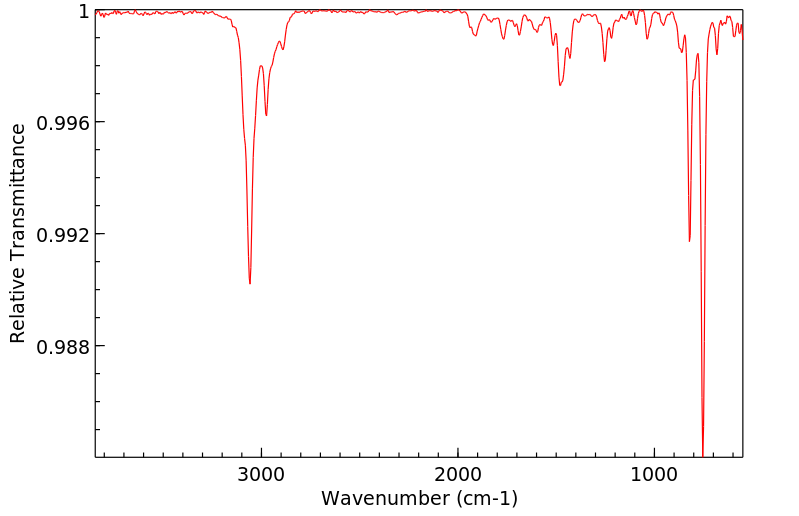
毒理性
国际癌症研究机构致癌物:4-甲基屈艿
IARC Carcinogenic Agent:4-Methylchrysene
来源:International Agency for Research on Cancer (IARC)