1,1,3,3-tetrachloropropanol-2 | 18992-39-7
中文名称
——
中文别名
——
英文名称
1,1,3,3-tetrachloropropanol-2
英文别名
1,1,3,3-tetrachloro-propan-2-ol;β.β.β'.β'-Tetrachlor-isopropylalkohol;1,1,3,3-Tetrachlor-propan-2-ol;1,1,3,3-Tetrachlor-isopropylalkohol;1,1,3,3-Tetrachloro-2-propanol;2-Propanol, 1,1,3,3-tetrachloro-;1,1,3,3-tetrachloropropan-2-ol
CAS
18992-39-7
化学式
C3H4Cl4O
mdl
——
分子量
197.876
InChiKey
DJQBSIUDOQUMHD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:34.5-35.5 °C
-
沸点:283.97°C (rough estimate)
-
密度:1.6120
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1,1,1,3,3,3-hexachloro-propan-2-ol 6188-63-2 C3H2Cl6O 266.766
反应信息
-
作为反应物:参考文献:名称:Dialkyl hydrogen phosphites and dialkyl phosphorochloridites derived from chlorine-substituted alcohols摘要:DOI:10.1007/bf01151360
-
作为产物:参考文献:名称:Neunhoeffer,O.; Spange,A., Justus Liebigs Annalen der Chemie, 1960, vol. 632, p. 22 - 27摘要:DOI:
文献信息
-
New bicyclic compounds as crac channel modulators申请人:Almirall, S.A.公开号:EP2738172A1公开(公告)日:2014-06-04The present invention relates to novel compounds which are inhibitors of CRAC channel activity. This invention also relates to pharmaceutical compositions containing them, process for their preparation and their use in therapy.这项发明涉及一种新型化合物,它们是CRAC通道活性的抑制剂。该发明还涉及含有这些化合物的药物组合物,它们的制备方法以及它们在治疗中的应用。
-
NEOPETROSIDES A AND B, AND SYNTHESIS METHOD THEREOF申请人:INJE UNIVERSITY INDUSTRY-ACADEMIC COOPERATION FOUNDATION公开号:US20190218206A1公开(公告)日:2019-07-18The present invention relates to novel pyridine nucleoside compounds, Neopetroside A (NPS A) and Neopetroside B (NPS B) obtained by fractionating an extract of Neopetrosia sp. which is a marine sponge, with ethanol, into n-butanol (n-BuOH). When the NPS A was treated with myocardial cells, it activates oxidative phosphorylation, basal and mitochondrial respiration induced by ATP and ATP synthesis, and stimulates the glycolysis activity and when the NPS A was treated with an ischemic reperfusion injury model, the left ventricular pressure injured by ischemic reperfusion was recovered and the size of myocardial injury site was significantly reduced and as a result, can be provided as the pharmaceutical composition for preventing or treating ischemic heart disease or the health functional food for preventing or improving the same.
-
4-bromo or 4-iodo phenylamino benzhydroxamic acid derivatives and their use as MEK inhibitors申请人:——公开号:US20030078428A1公开(公告)日:2003-04-24Phenylamino benzhydroxamic acid derivatives of formula (I) where R 1 , R 2 , R 3 , R 4 , R 5 , and R 6 are hydrogen or substituent groups such as alkyl, and where R 7 is hydrogen or an organic radical, are potent inhibitors of MEK and, as such, are effective in treating cancer and other proliferative diseases such as psoriasis and restenosis.
-
[EN] 1,2,3,6-TETRAHYDROAZEPINO[4,5-B]INDOLE-5-CARBOXYLATE NUCLEAR RECEPTOR INHIBITORS<br/>[FR] INHIBITEURS DES RÉCEPTEURS NUCLÉAIRES DE 1,2,3,6-TÉTRAHYDROAZÉPINO[4,5-B]INDOLE-5-CARBOXYLATE申请人:WYETH CORP公开号:WO2010036362A1公开(公告)日:2010-04-01Provided are certain 1,2,3,6- tetrahydroazepino[4,5-b]indole-5-carboxylate compounds which are useful for modulating the activity of nuclear receptors, such as farnesoid X receptors, and/or for the treatment, prevention, or amelioration diseases or disorders related to the activity of these receptors.提供了一些1,2,3,6-四氢噁二氮杂并[4,5-b]吲哚-5-羧酸酯化合物,这些化合物对调节核受体的活性(如法尼索X受体)非常有用,或者用于治疗、预防或改善与这些受体活性相关的疾病或疾病。
-
[EN] 4-BROMO OR 4-IODO PHENYLAMINO BENZHYDROXAMIC ACID DERIVATIVES AND THEIR USE AS MEK INHIBITORS<br/>[FR] DERIVES D'ACIDE BENZHYDROXAMIQUE PHENYLAMINO 4-BROMO OU 4-IODO ET UTILISATION DE CES DERNIERS EN TANT QU'INHIBITEURS DE MEK申请人:WARNER-LAMBERT COMPANY公开号:WO1999001426A1公开(公告)日:1999-01-14(EN) Phenylamino benzhydroxamic acid derivatives of formula (I) where R1, R2, R3, R4, R5, and R6 are hydrogen or substituent groups such as alkyl, and where R7 is hydrogen or an organic radical, are potent inhibitors of MEK and, as such, are effective in treating cancer and other proliferative diseases such as psoriasis and restenosis.(FR) On décrit des dérivés d'acide benzhydroxamique phénylamino de formule (I). Dans la formule R1, R2, R3, R4, R5 et R6 représentent hydrogène ou des groupes substituants tels que alkyle; R7 représentant hydrogène ou un radical organique. Ces dérivés sont de puissants inhibiteurs de MEK et en tant que tels ils sont efficaces dans le traitement du cancer et d'autres maladies proliférantes telles que le psoriasis et la resténose.Phenylamino benzhydroxamic acid derivatives of formula (I) where R1, R2, R3, R4, R5, and R6 are hydrogen or substituent groups such as alkyl, and where R7 is hydrogen or an organic radical, are potent inhibitors of MEK and, as such, are effective in treating cancer and other proliferative diseases such as psoriasis and restenosis. 翻译:公式(I)中的苯氨基苯甲酰羟肟酸衍生物,其中R1、R2、R3、R4、R5和R6是氢或取代基,例如烷基,而R7是氢或有机基团,是MEK的强效抑制剂,因此在治疗癌症和其他增生性疾病,如银屑病和再狭窄方面非常有效。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
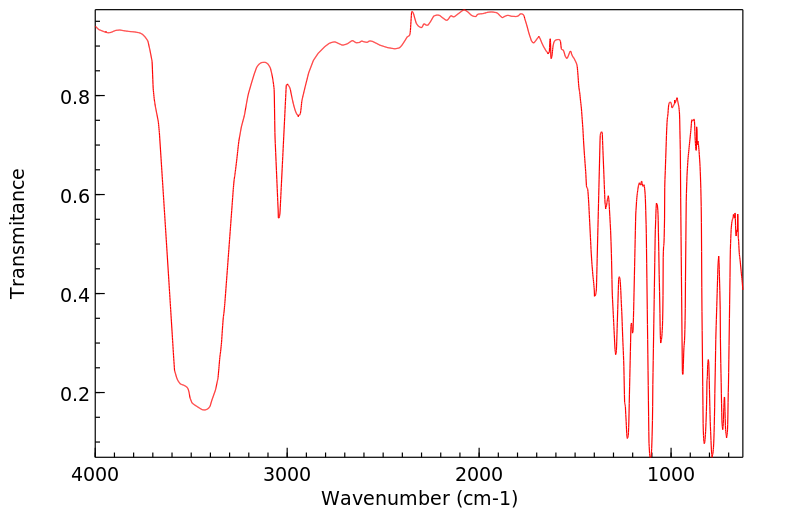
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺-(1S,2S)-1,2-二氢-3-氟邻苯二酚
苏-3-溴-2-丁醇
苏-3-溴-2-丁醇
烯丙基3-氯-2-羟基丙酸酯
溶剂紫36
溴丁醇
水合氯醛
氯醛甜菜碱
氯醛叔丁基半缩醛
氯醛丙基半缩醛
氯二氟乙醛’水合物
氯-(2-氯-3-羟基丙-1-烯基)汞
氘代3-氯-1,2-丙二醇
培氟沙星
四氟乙醇
四氟丙醇
四氟丁二醇
十二氟庚醇
十一氟正己烷-1-醇
六氟异丙醇
六氟丁醇
六氟-1-丙醇
八氟代-1-戊醇
八氟-1,6-己二醇
全氟十醇
全氟-1-辛醇
全氟-1-庚醇
五氟丙醛甲基半缩醛
五氟丙醛水合物
五氟丙醛乙基半缩醛
二溴甘露醇
二氯乙醛水合物
二氯乙氧基合氧钒
二氟乙醛缩半乙醇
乙基3-氟-2-羟基-3-甲基丁酸酯
三溴乙醇
三氟甲基己醇
三氟乙醛缩甲基半醇
三氟乙醛水合物
三氟乙醇
三氟乙基醇-OD
七氟丁醛乙基半缩醛
丁氯醇
rac-2-氯十二烷-1-醇
rac-1-氯十二烷-2-醇
alpha,alpha-二(三氟甲基)-1-氮丙啶甲醇
[2H4]-2-溴-1,3-丙二醇[干冰运输]
[1-氯-3-异丙基氨基-2-丙醇
[1,1-(2)H2]-2-氯乙醇
O-(1,1,3-三氢四氟丙基)-(1-羟基-2,2,2-三氯乙基)甲基膦酸酯