5,6-二甲基癸烷 | 1636-43-7
中文名称
5,6-二甲基癸烷
中文别名
——
英文名称
5,6-dimethyldecane
英文别名
5.6-Dimethyl-decan
CAS
1636-43-7
化学式
C12H26
mdl
——
分子量
170.338
InChiKey
NCJIZIYQFWXMFZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-50.8°C (estimate)
-
沸点:201°C
-
密度:0.7567
-
保留指数:1135
计算性质
-
辛醇/水分配系数(LogP):6.1
-
重原子数:12
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901100000
SDS
反应信息
-
作为产物:参考文献:名称:Muedas, Cesar A.; Ferguson, Richard R.; Brown, Stephen H., Journal of the American Chemical Society, 1991, vol. 113, # 6, p. 2233 - 2242摘要:DOI:
文献信息
-
1-Hexene Oligomerization in Liquid, Vapor, and Supercritical Phases over Beidellite and Ultrastable Y Zeolite Catalysts作者:Jérôme P.G. Pater、Pierre A. Jacobs、Johan A. MartensDOI:10.1006/jcat.1998.2250日期:1998.10zeolite catalysts. Vapor, liquid, and supercritical states of the reacting hydrocarbons in the reactor tube were established by using propane, pentane, octane, and dodecane as solvents. The initial activity, stability with operation time, and selectivity are very dependent on the physical state of the hydrocarbons. Highest activity and stability are reached in the liquid phase using octane and dodecane solvent
-
Ultrasound Assisted Synthesis of 5,9-Dimethylpentadecane and 5,9-Dimethylhexadecane – the Sex Pheromones of Leucoptera coffeella作者:Nhuan Doan、Thach Le、Hao Nguyen、Poul Hansen、Fritz DuusDOI:10.3390/12082080日期:——Racemic 5,9-dimethylpentadecane and 5,9-dimethylhexadecane, the major and minor constituents, respectively, of the sex pheromone of Leucoptera coffeella, have been synthesized from citronellol in 56-58% overall yield through six steps. Ultrasound irradiation efficiently supported tosylation of alcohols (two steps) as well as the subsequent cross coupling reactions with the pertinent Grignard reagents (also two steps).
-
Formation of Three-Membered Rings by S<sub>H</sub>i Displacement. Reverse of Cyclopropyl Ring Opening<sup>1</sup>作者:Dennis D. Tanner、Liying Zhang、Li Qing Hu、Pramod KandanarachchiDOI:10.1021/jo960879u日期:1996.1.1carried out in the presence of several transfer agents (CCl(4), CCl(3)Br, CCl(2)Br(2)) initiate a radical chain addition of CCl(2)X(*) and yield cyclized materials resulting from the S(H)i displacement of halogen by a carbon-centered radical. The radical displacement of a halogen on carbon, the reverse of homolytic displacement on cyclopropyl carbon, is dominant at low temperatures. The rate constants for通常的方法是将卤甲烷光引发或过氧化物引发的自由基甲烷加成烯烃,在20至100摄氏度的温度范围内产生1,2-加成产物。在较低的温度(-42至-104摄氏度)下,竞争性反应加入CCl(2)X(*)之后,生成烷基环丙烷。1-辛烯或1-己烯和1-甲基环己烯与氢原子的反应在几种转移剂(CCl(4),CCl(3)Br,CCl(2)Br(2))的存在下进行CCl(2)X(*)的链加成并产生环化的材料,该环化的材料是由碳中心自由基取代卤素的S(H)i。在低温下,卤素在碳上的自由基置换与环丙基碳上的均质置换相反。环化速率常数(k(c))与卤代甲烷转移速率常数(k(t))的等速动力学温度为-46摄氏度(CCl(4),1-己烯);-35摄氏度(CCl(4),1-甲基环己烯)。在BrCCl(3)存在下进行的两种底物反应的等动力学温度计算为-204摄氏度(1-辛烯)和-109摄氏度(1-甲基环己烯)。
-
Addition reactions of organometallic reagents to nitrogen trifluoride and enhanced alkyl–alkyl coupling by NF3作者:Randolph K. BelterDOI:10.1016/j.jfluchem.2015.03.013日期:2015.7A survey of the reaction of nitrogen trifluoride (NF3) with various organometallic reagents finds that organomagnesium (Grignard) reagents are the most useful for producing N,N-difluoroaminoalkanes. Alkyl–alkyl coupling is a persistant side reaction. Organolithiums are marginally effective. Organocopper, organozinc reagents undergo primarily alkyl–alkyl coupling catalyzed by the presence of NF3. Organocalcium
-
TaCl5-catalyzed reaction of 1-alkenes with n-alkyl Grignard reagents作者:Rifkat M. Sultanov、Ruslan R. Ismagilov、Natal'ya R. Popod'ko、Artur R. Tulyabaev、Usein M. DzhemilevDOI:10.1016/j.jorganchem.2012.10.001日期:2013.1Tantalum-catalyzed reaction of organomagnesium compounds (OMCs), bearing normal alkyl radicals, with 1-alkenes to afford novel iso-alkylmagnesiums has been systematically studied for the first time. The probable mechanism of the reaction implying the intermediate formation of β,β′-dialkyl substituted tantalacyclopentanes is discussed.
表征谱图
-
氢谱1HNMR
-
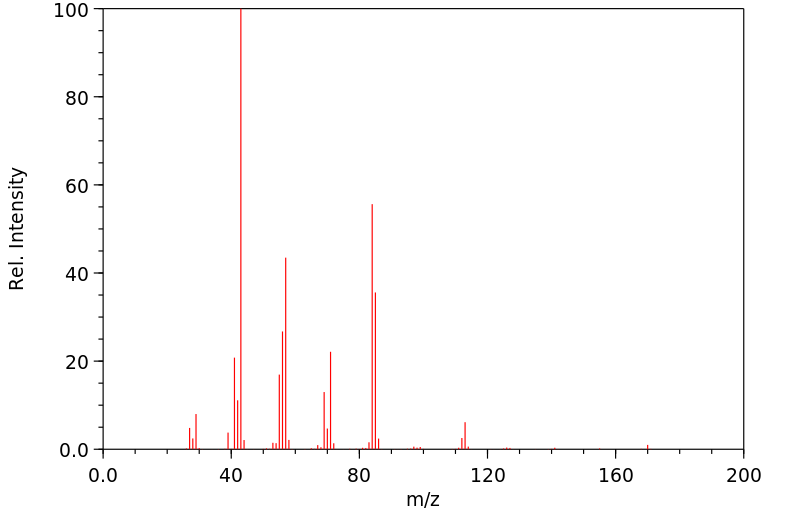
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1-乙基-3-甲基环己烷
顺式-1-乙基-2-甲基环丙烷
顺式-1,3-二甲基环庚烷
顺式-1,2-二甲基环丙烷
顺式-1,2-二乙基环戊烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式,反式,反式-1,2,4-三甲基环己烷
Copper, ethyl-
辛烷-d18
辛基环戊烷
辛基环丙烷
联苯肼酯
联环戊基
羰基双(环茂二烯基)钛
矿油精
癸烷,2,8-二甲基-
癸烷
decyl radical
癸基环戊烷
異十八烷
甲烷-d3
甲烷-d2
甲烷-d1
甲烷-D4
甲烷-3H
甲烷-13C,d4
甲烷-13C
甲烷
甲基自由基
甲基环辛烷
甲基环癸烷
甲基环戊烷
甲基环己烷-Me-d3
甲基环己烷
甲基环十一烷
甲基环丙烷
甲基环丁烷.
甲基丙烷-2-d
环辛烷-D16
环辛烷
环癸烷
环戊烷-D9
环戊烷-D10
环戊烷-13C1
环戊烷,三(2-辛基十二基)-
环戊烷
环戊基甲基自由基
环戊基环庚烷
环戊基环己烷