5,7-二羟基-3’,4’,5’-三甲氧基黄烷酮 | 62252-10-2
中文名称
5,7-二羟基-3’,4’,5’-三甲氧基黄烷酮
中文别名
5,7-DIHYDROXY-3′,4′,5′-TRIMETHOXYFLAVANONE5,7-二羟基-3',4',5'-三甲氧基黄烷酮;5,7-二羟基-3',4',5'-三甲氧基黄烷酮
英文名称
5,7-dihydroxy-3',4',5'-trimethylhydroxyflavone
英文别名
5,7-dihydroxy-3',4',5'-trimethoxyflavanone;5,7-dihydroxy-2-(3,4,5-trimethoxy-phenyl)-chroman-4-one;5,7-Dihydroxy-2-(3,4,5-trimethoxy-phenyl)-chroman-4-on;5,7-Dihydroxy-3',4',5'-trimethoxy-flavanon;5,7-dihydroxy-2-(3,4,5-trimethoxyphenyl)-2,3-dihydrochromen-4-one
CAS
62252-10-2
化学式
C18H18O7
mdl
——
分子量
346.337
InChiKey
CTALFFOVOLJORS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:227-230°C
-
LogP:3.370 (est)
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:25
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.28
-
拓扑面积:94.4
-
氢给体数:2
-
氢受体数:7
安全信息
-
海关编码:2932999099
SDS
反应信息
-
作为反应物:描述:5,7-二羟基-3’,4’,5’-三甲氧基黄烷酮 、 2,3,4-三-O-乙酰基-α-D-木吡喃糖苷溴 在 盐酸 、 lithium 作用下, 生成 7-O-xylosyl-4'-O-methyltricin参考文献:名称:通过合成鉴定天然三黄酮衍生的 C-糖苷摘要:摘要 5,7-二羟基-3',4',5'-三甲氧基黄酮的C-糖基化与乙酰溴-α-d-葡萄糖、-α-d-半乳糖、-α-d-木糖、-β- l-阿拉伯糖和-αt-L-鼠李糖。分离并全甲基化各自的 6-C-糖苷和 6, 8-二-C-糖苷(半乳糖除外)。MS 和 TLC 比较证实了五种天然三糖苷衍生的 C-糖苷的拟议结构。DOI:10.1016/0031-9422(83)80167-8
-
作为产物:参考文献:名称:10.1002/cmdc.202400056摘要:Neuroinflammation is an inflammatory immune response that arises in the central nervous system. It is one of the primary causes of neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease. Phloroglucinol (PG) is a natural product contained in extracts of plant, algae and microbe and has been reported to have antioxidant and anti‐inflammatory properties. In this study, we synthesized PG derivatives to enhance their antioxidant and anti‐inflammatory activity. Among PG derivatives, 6a suppressed pro‐oxidative and inflammatory molecule nitric oxide (NO) production more effectively than PG. Moreover, 6a dose‐dependently reduced the expression of proinflammatory cytokines such as IL‐6, IL‐1β, TNF‐α, and NO producing enzyme iNOS in lipopolysaccharide (LPS)‐stimulated BV‐2 microglial cells. Additionally, we confirmed that 6a alleviated cognitive impairment and glial activation in mouse model of LPS‐induced neuroinflammation. These findings suggest that novel PG derivative, 6a, is a potential treatment for neurodegenerative diseases.DOI:10.1002/cmdc.202400056
文献信息
-
Methods For Treating Wounds申请人:Kimberly-Clark Worldwide, Inc.公开号:US20030125264A1公开(公告)日:2003-07-03Pharmaceutical compositions, and methods of using the same, are provided utilizing effective amounts of one or more flavones, flavonols, flavanones, isoflavanones and isoflavones to increase cell proliferation in various tissues and cell lines. As examples, the composition and methods of the present invention can be used to increase proliferation of fibroblast cells and, more particularly, in the treatment of wounds as well as strengthening of the skin.
-
Shinoda et al., Yakugaku Zasshi/Journal of the Pharmaceutical Society of Japan, 1931, vol. 51, p. 249,252; dtsch. Ref. S. 23作者:Shinoda et al.DOI:——日期:——
-
Compounds exhibiting efflux inhibitor activity and composition and uses thereof申请人:Wempe Fitzpatrick Michael公开号:US20070254859A1公开(公告)日:2007-11-01At least one compound chosen from compounds of Formula I: a pharmaceutically acceptable salt or ester thereof, a solvate thereof, a chelate thereof, a non-covalent complex thereof, a prodrug thereof, and mixtures of any of the foregoing, wherein: n is a number from 1 to 900, wherein the individual units may be the same or different; W is chosen from alkyl, substituted alkyl, cycloalkyl, substituted cycloalkyl, aryl, substituted aryl, aralkyl and substituted aralkyl; each of R 2 , R 3 , R 4 and R 5 is independently chosen from —H, alkyl, substituted alkyl, cycloalkyl, substituted cycloalkyl, aryl, substituted aryl, aralkyl and substituted aralkyl; Z′ is chosen from —O—, —N—, —NO—, —NR 4 —, —S—, —SO— and —SO 2 —, wherein R 4 is defined as above; each of X, X′, Y and Z is independently chosen from —CR 4 R 5 —, —NH—, —NR 4 —, —NO—, —O—, —NOR 4 —, —S—, —SO—, —SO 2 —, wherein R 4 and R 5 are defined as above; R 1 is chosen from a tocopherol, a steroid and a flavonoid; and R 6 is chosen from any R 1 , alkyl, substituted alkyl, cycloalkyl, substituted cycloalkyl, aryl, substituted aryl, aralkyl and substituted aralkyl.
-
NOX INHIBITOR AND NFkB INHIBITOR CONTAINING METHOXYFLAVONE申请人:SUNTORY HOLDINGS LIMITED公开号:US20170071902A1公开(公告)日:2017-03-16The present invention aims at providing NOX inhibitors and NFκB inhibitors having superior actions, as well as agents for preventing or treating NOX- or NFκB-associated diseases that utilize such inhibitors. To this end, specified methoxyflavones are employed.
-
[EN] COMPOSITIONS AND METHODS FOR INCREASING CELL PROLIFERATION COMPRISING A FLAVONOID<br/>[FR] COMPOSITIONS ET METHODES D'AUGMENTATION DE LA PROLIFERATION CELLULAIRE CONTENANT UN FLAVONOIDE申请人:KIMBERLY CLARK CO公开号:WO2003057210A1公开(公告)日:2003-07-17Pharmaceutical compositions, and methods of using the same, are provided utilizing effective amounts of one or more flavones, flavonols, flavanones, isoflavanones and isoflavones to increase cell proliferation in various tissuesand cell lines. As examples, the composition and methods of the present invention can be used to increase proliferation of fibroblast cells and, more particularly, in the treatment of wounds as well as strengthening of the skin.
表征谱图
-
氢谱1HNMR
-
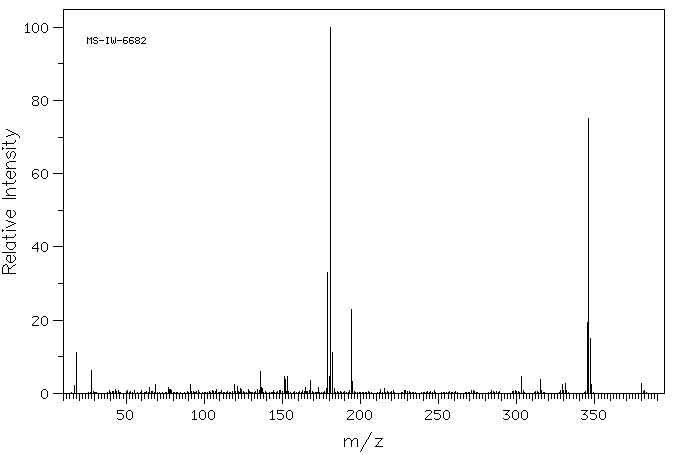
质谱MS
-
碳谱13CNMR
-
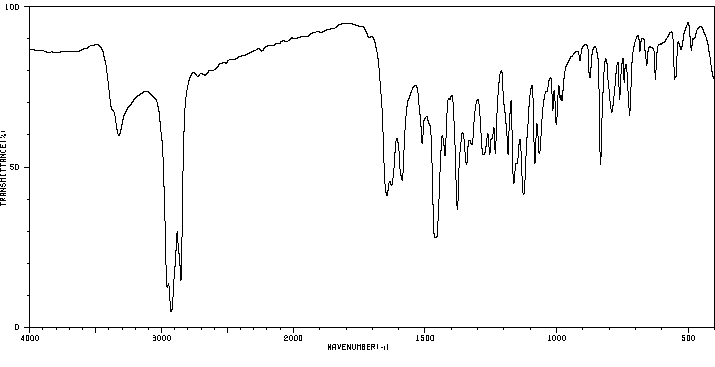
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
([2-(萘-2-基)-4-氧代-4H-色烯-8-基]乙酸)
龙血树脂红血树脂
鼠李素
鼠李柠檬素3-O-beta-D-鼠李三糖苷
鼠李柠檬素
鼠李亭3-O-beta-吡喃葡萄糖苷
黄酮醇-2-磺酸钠盐
黄酮胺
黄酮榕碱
黄酮地洛
黄酮哌酯
黄酮
黄诺马甙
黄苏木素
黄花夹竹桃黄酮
黄芪总皂甙
黄芩黄酮II
黄芩黄酮I
黄芩黄酮
黄芩苷甲酯
黄芩苷
黄芩素磷酸酯
黄芩素一水合物
黄芩素-7-甲醚
黄芩素 6-O-beta-D-吡喃葡萄糖苷
黄芩素
黄烷酮腙
黄烷酮-d5
黄烷酮
黄杞苷
黄宝石羽扇豆素
麗春花青苷
鳞叶甘草素B
高车前苷
高车前素-4'-O-Β-D-葡萄糖苷
高车前素
高良姜素-5-甲基醚
高良姜素-3-甲基醚
高良姜素
高圣草酚-7-O-(6''-O-乙酰基)吡喃葡萄糖苷
高圣草酚
高圣草素-7-O-Β-D-葡萄糖苷
高圣草素
骨碎补素
马里甙
马醉木素
马缨丹黄酮苷
马来酸2-乙酰基-10-[2-(二甲基氨)丙基]-10H-苯并噻嗪正离子
香风草甙
香蒲新苷