potassium isethionate | 1561-99-5
中文名称
——
中文别名
——
英文名称
potassium isethionate
英文别名
Potassium 2-hydroxyethanesulfonate;potassium;2-hydroxyethanesulfonate
CAS
1561-99-5
化学式
C2H5O4S*K
mdl
——
分子量
164.224
InChiKey
DMNPUBKRNRRYMC-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:191-193°C
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):-4.47
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:85.8
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险品标志:Xn
-
安全说明:S22,S24/25,S36
-
危险类别码:R40
-
海关编码:2905590090
-
储存条件:密封储存,应存放在阴凉、干燥的库房中。
SDS
| Name: | Isethionic Acid Potassium Salt 98% (Titr.) Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 1561-99-5 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1561-99-5 | Ethanesulfonic Acid, 2-Hydroxy-, Monop | 98 | 216-342-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1561-99-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C2H5KO4S
Molecular Weight: 164.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide, oxides of potassium.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1561-99-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Ethanesulfonic Acid, 2-Hydroxy-, Monopotassiumsalt - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1561-99-5: No information available.
Canada
CAS# 1561-99-5 is listed on Canada's DSL List.
CAS# 1561-99-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1561-99-5 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:potassium isethionate 生成 alkaline earth salt of/the/ methylsulfuric acid参考文献:名称:Kohler, American Chemical Journal, 1898, vol. 20, p. 692摘要:DOI:
-
作为产物:描述:参考文献:名称:The Addition of Sodium Bisulfite to Alkylene Oxides摘要:DOI:10.1021/ja01301a014
文献信息
-
N-Sulfo alkane amino alkane phosphoric acids and their alkali metal申请人:Benckiser-Knapsack GmbH公开号:US04216163A1公开(公告)日:1980-08-05Novel and highly advantageous N-sulfo alkane amino alkane phosphonic acids and their alkali metal salts are provided. Said phosphonic acids are produced by reacting an alkali metal salt of an amino phosphonic acid with a halo, preferably chloro, or hydroxy alkane sulfonic acid or their alkali metal salts in an alkaline medium, while heating. In place of the halo and especially chloro or hydroxy alkane sulfonic acid reactants, there can also be used compounds which are capable of producing hydroxy alkane sulfonates such as carbylsulfate or aldehydes or, respectively, ethylene oxide with alkali metal bisulfites or metasulfites. The reaction is preferably carried out in a molar proportion of about 1:1 to about 1:2. The novel compounds are excellent complexing or sequestering agents especially with respect to polyvalent metal ions. They are highly resistant against hydrolysis and high temperatures and are of a very high water solubility.
-
一种直接法合成脂肪酰基氨基酸表面活性剂的方法申请人:张家港格瑞特化学有限公司公开号:CN111004156A公开(公告)日:2020-04-14
-
Synthesis of 2-Substituted N-Nitrooxazolidines作者:A.S. Ermakov、E.Yu. Petrov、Yu.A. Strelemko、D.B. Vinogradov、V.A. TartakovskiiDOI:10.1007/s11178-005-0315-7日期:2005.8A method was developed for preparation of N-nitrooxazolidines functionally substituted in position 2 consisting in nitration of reaction products obtained from N-(2-hydroxyethyl)sulfamate and 2-substituted acetaldehydes.
-
66. Reactions of ethylene oxides. Part II. Reactions with thioamides, thiols, and inorganic sulphur salts作者:C. C. J. Culvenor、W. Davies、N. S. HeathDOI:10.1039/jr9490000278日期:——
-
Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: S: MVol.B2, 7.6.2, page 953 - 957作者:DOI:——日期:——
表征谱图
-
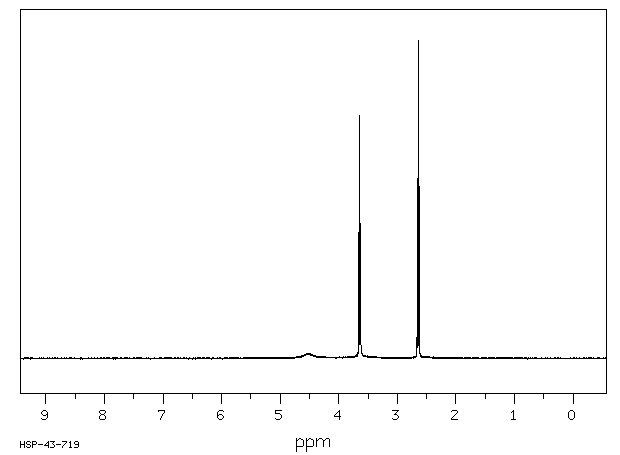
氢谱1HNMR
-
质谱MS
-
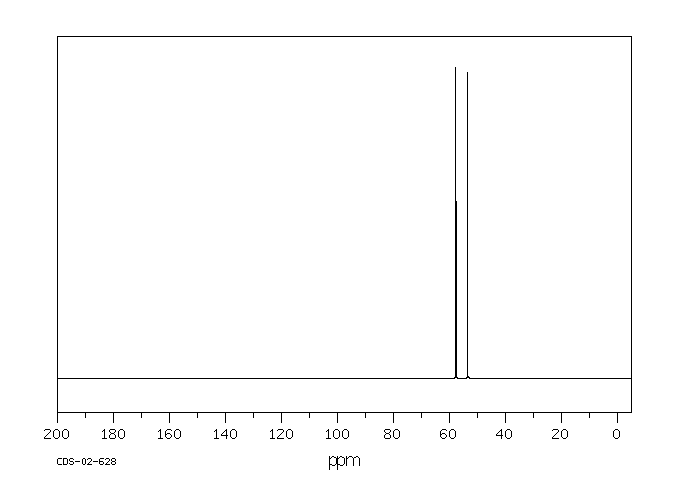
碳谱13CNMR
-
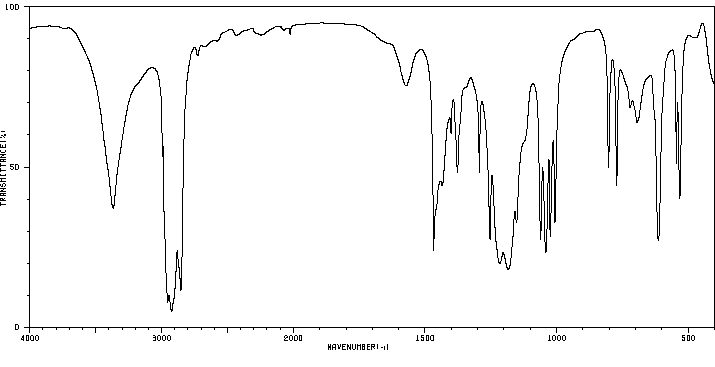
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高烯丙基(甲磺酰基)胺
高炔丙基(甲磺酰基)胺
顺式-9-十八碳烯基甲烷磺酸酯
顺式-4-乙基环己基甲烷磺酸酯
顺-1,2-双(甲磺酰基氧基甲基)环己烷
阿坎酸杂质
阿坎酸
锌甲烷磺酸盐
铵磺酸甜菜碱-3
铵磺酸甜菜碱-2
铵磺酸甜菜碱-1
铬雾抑制剂
铁三(三氟甲基磺酰基)亚胺
钾3-(三羟基硅烷基)-1-丙烷磺酸酯
钾1,1,2,2,3,3,4,4-八氟丁烷-1-磺酸盐
钡二乙烷磺酸酯
钠3-氨基丙烷磺酸酯
钠3-氨基-3-氧代-丙烷-1-磺酸酯
钠3-(三羟基硅烷基)-1-丙烷磺酸酯
钠2-[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-十八碳-9-烯氧基乙氧基)乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]乙氧基]-2-氧代-乙烷磺酸酯
钠1-羟基-1-庚烷磺酸酯
钠(1E)-1-十二碳烯-1-磺酸酯
酪朊酸钠
辛烷-1-磺酸甲酯
辛烷-1-磺酸乙酯
辛基-1-磺酸戊酯
辛-2-烯-1-磺酸
辅酶 M
西尼必利杂质7
萘-1,8-二甲醇
英丙舒凡对甲苯磺酸盐
英丙舒凡
苯基硒基三氟甲烷磺酸酯
芥酸酰胺丙基羟基磺基甜菜碱
艾日布林中间体
脒基牛磺酸
胺磺酸甜菜碱-4
联硫亚盐氯乙醛钠水合物
羧基-五聚乙二醇-磺酸
羟甲基磺酸钠
羟基甲烷磺酸铵盐
羟基甲烷磺酸钾
羟基甲氧基甲醇甲磺酸酯
羟乙磺酸钾
羟乙基磺酸铵盐
羟乙基磺酸钠
羟丙基硫代硫酸钠
美司那
磺酸钠
磺酸己烷