(E-)-N-methyl-1-(methylthio)-2-nitroethanamine | 61832-41-5
分子结构分类
中文名称
——
中文别名
——
英文名称
(E-)-N-methyl-1-(methylthio)-2-nitroethanamine
英文别名
(E)-N-methyl-1-(methylthio)-2-nitroethenamine;(E)-N-methyl-1-(methylsulfanyl)-2-nitroethenamine;NMSM;(E)-N-methyl-1-(methylsulfanyl)-2-nitroethen-1-amine;1-methylthio-1-methylamino-2-nitroethylene;Ethenamine, N-methyl-1-(methylthio)-2-nitro-;(E)-N-methyl-1-methylsulfanyl-2-nitroethenamine
CAS
61832-41-5
化学式
C4H8N2O2S
mdl
——
分子量
148.186
InChiKey
YQFHPXZGXNYYLD-ONEGZZNKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:112-118 °C (lit.)
-
沸点:236.5±35.0 °C(Predicted)
-
密度:1.199±0.06 g/cm3(Predicted)
-
溶解度:可溶于氯仿、甲醇(少量)
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:83.2
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2930909090
-
危险品运输编号:UN 3335
-
包装等级:III
-
危险类别:9
-
危险性防范说明:P261,P264,P271,P280,P302+P352,P304+P340,P305+P351+P338,P310,P362+P364,P403+P233,P501
-
危险性描述:H315,H318,H335,H411
SDS
反应信息
-
作为反应物:描述:(E-)-N-methyl-1-(methylthio)-2-nitroethanamine 在 ammonium hydroxide 作用下, 以 乙醇 为溶剂, 反应 4.0h, 以63%的产率得到(Z)-1-N'-methyl-2-nitroethene-1,1-diamine参考文献:名称:合成这些初级硝基酮胺摘要:Die Titelverbindungen 3 和 4 lassen sich sowohl aus dem Nitroketen-O,N-acetal 1 und prim。呜呜呜 塞孔德。Aminen als auch durch sukzessive Amino- und Ammonolyse des Nitroketen-S,S-acetals 2 gewinnen。Die Reaktion von 2 mit wäßrigem Ammoniak führt zum dimeren Nitroacetonitril 9.DOI:10.1002/ardp.19863190213
-
作为产物:参考文献:名称:Process for preparing 2-amino-2-alkylthio-1-nitroethylene compounds摘要:通过将1-较低烷基磺酰基-1-较低烷基硫-2-硝基乙烯与胺反应制备2-氨基-2-烷基硫-1-硝基乙烯化合物的方法。该过程的产物是生产组织胺H.sub.2 -拮抗剂的中间体。公开号:US04028379A1
-
作为试剂:参考文献:名称:Four-Component Domino Strategy for the Combinatorial Synthesis of Novel 1,4-Dihydropyrano[2,3-c]pyrazol-6-amines摘要:An efficient one-pot four-component domino protocol for the combinatorial synthesis of novel 1,4-dihydropyrano[2,3-c]pyrazol-6-amines has been achieved. This transformation presumably occurs via cyclization- Knoevenagel condensation-Michael addition-tautomerism- intramolecular O-cyclization-elimination sequence of reactions. The significant features of this reaction include expedient one-pot process, short reaction time, no column chromatographic purification, excellent yield, and readily available starting materials.DOI:10.1021/co4000997
文献信息
-
[EN] ANTIMICROBIAL [3.1.0] BICYCLOHEXYLPHENYL-OXAZOLIDINONE DERIVATIVES AND ANALOGUES<br/>[FR] DERIVES DE [3.1.0] BICYCLOHEXYLPHENYL-OXAZOLIDINONE ANTIMICROBIENS ET LEURS ANALOGUES申请人:UPJOHN CO公开号:WO2004089943A1公开(公告)日:2004-10-21The present invention provides certain [3.1.0] bicyclic oxazolidinone derivatives of Formula (I) or pharmaceutically acceptable salts or prodrugs thereof that are antibacterial agents, pharmaceutical compositions containing them, methods for their use, and methods for preparing these compounds.
-
One-pot pseudo three-component reaction of nitroketene-N,S-acetals and aldehydes for synthesis of highly functionalized hexa-substituted 1,4-dihydropyridines作者:H. Surya Prakash Rao、A. ParthibanDOI:10.1039/c4ob00628c日期:——We have described the simple, convenient and high yielding one-pot synthesis of a library of highly functionalized hexa-substituted 1,4-dihydropyridines (1,4-DHPs) by 2-aminopyridine catalysed pseudo three-component reaction of nitroketene-N,S-acetals and aldehydes. This domino transformation involves formation of dihydropyridine ring by creation of two C–C bonds and one C–N bond, all of them taking
-
Dual active 1, 4-dihydropyridine derivatives: Design, green synthesis and in vitro anti-cancer and anti-oxidant studies作者:Parthiban Anaikutti、Parameshwar MakamDOI:10.1016/j.bioorg.2020.104379日期:2020.12The present work describes the design of 1,4-dihydropyridines (1,4-DHPs) with diverse variations in structural and functional groups. The physico-chemical properties and drug-like molecule nature evaluations were carried out using SWISSADME. A simple, economical, eco-friendly, water-mediated and Para-Toluene sulfonic acid catalysed multicomponent and one-pot synthetic method from nitroketene N, S-本工作描述了结构和功能基团具有多种变化的1,4-二氢吡啶(1,4-DHP)的设计。使用SWISSADME进行理化性质和类药物分子的性质评估。已经开发了一种简单,经济,环保,水介导的对甲苯磺酸催化的多硝基硝基烯N,S-乙缩醛(NMSM)和相应醛类的多锅法合成方法。所有化合物(6a-u和13a-h)都针对两种重要的人类癌细胞系Viz进行了体外测定。是喉癌(Hep2)和肺腺癌(A549)细胞。DPPH的降低水平(%),用于评估抗氧化性能。1,4-DHP衍生物6o,6u和6l显示出有效的抗癌活性,对Hep2的IC 50值为10 µM,14 µM和10 µM,对A549细胞的IC 50值为8 µM,9 µM和50 µM。同样,标准浓度为50 µg时6o,6l和6u的抗氧化性能分别为70.12%,63.90%和59.57%,有利于1,4-DHP衍生物的双重活性。发现化合物6o和6l与标准药物阿霉素相当。
-
One-pot practical method for synthesis of functionalized 4<i>H</i>-chromen-5-one derivatives under catalyst and solvent-free conditions作者:M. Musawwer Khan、Biswas Saigal、Sumbulunnisan Shareef、Sarfaraz Khan、Subash C. SahooDOI:10.1080/00397911.2018.1517218日期:2018.10.18Abstract A facile and practical method was described for the synthesis of 4H-chromen-5-ones under catalyst- and solvent-free conditions by one-pot stirring of starting materials at 110 °C. The products were obtained by the reaction between cyclic 1,3-dicarbonyl compounds, aromatic aldehydes and (E)-N-methyl-1-(methylthio)-2-nitroethenamine (NMSM) in short duration with good to excellent yields. This
-
Thiamine analogues and their use as antibiotics申请人:Rheinische Friedrich-Wilhelms-Universität Bonn公开号:EP2626356A1公开(公告)日:2013-08-14The present invention relates to compounds according to general formula (I), pharmaceutical compositions comprising compounds according to general formula (I) and the use of the compounds for the treatment of a bacterial infection, particularly for use as an antibiotic.这项发明涉及符合一般式(I)的化合物,包括含有符合一般式(I)的化合物的药物组合物,以及利用这些化合物治疗细菌感染,特别是用作抗生素的用途。
表征谱图
-
氢谱1HNMR
-
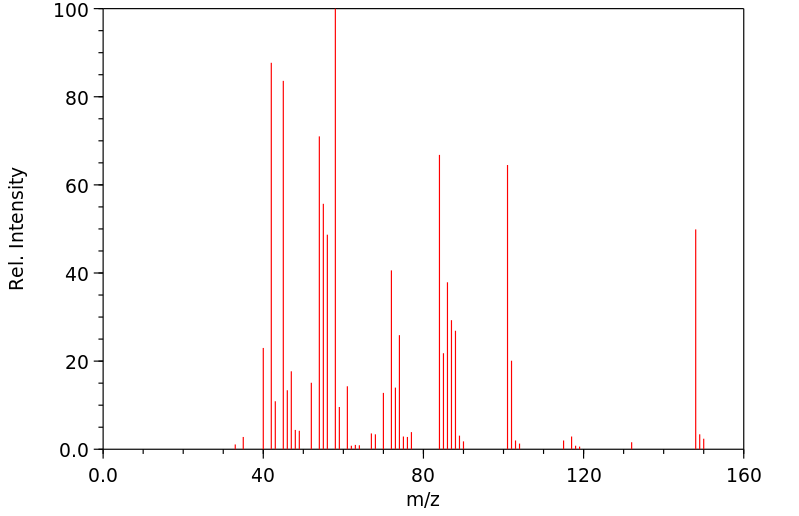
质谱MS
-
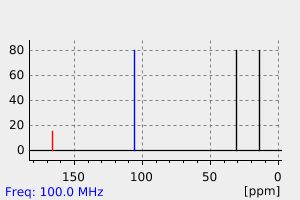
碳谱13CNMR
-
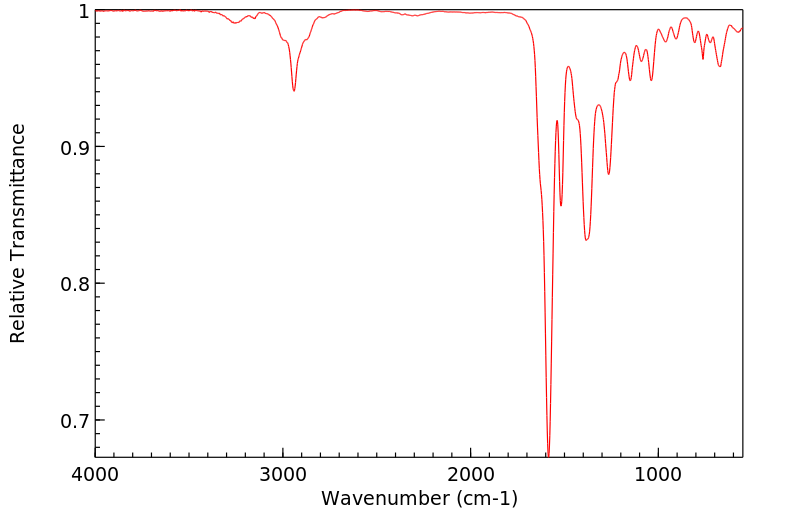
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-硝基环己基乙酸酯
顺式-2-硝基-6-甲基环己酮
雷尼替丁杂质18
铝硝基甲烷三氯化物
钾离子载体III
重氮(硝基)甲烷
醛基-七聚乙二醇-叠氮
过氧亚甲基
辛腈,4-氟-4-硝基-7-羰基-
辛烷,1,2-二氯-1-硝基-
赤霉素A4+7(GA4:GA7=65:35)
苄哒唑
羟胺-四聚乙二醇-叠氮
羟胺-三乙二醇-叠氮
米索硝唑
磷酸十二醇酯
碘硝基甲烷
碘化e1,1-二甲基-4-羰基-3,5-二(3-苯基-2-亚丙烯基)哌啶正离子
硝酰胺
硝基脲银(I)复合物
硝基甲醇
硝基甲烷-d3
硝基甲烷-13C,d3
硝基甲烷-13C
硝基甲烷-(15)N
硝基甲烷
硝基甲基甲醇胺
硝基环辛烷
硝基环戊烷
硝基环戊基阴离子
硝基环庚烷
硝基环己烷锂盐
硝基环己烷钾盐
硝基环己烷
硝基环丁烷
硝基氨基甲酸
硝基新戊烷
硝基二乙醇胺
硝基乙醛缩二甲醇
硝基乙醛缩二乙醇
硝基乙腈
硝基乙烷-D5
硝基乙烷-1,1-d2
硝基乙烷
硝基乙烯
硝基丙烷
硝基丙二醛(E,E)-二肟
硝基丙二腈
硝基-(3-硝基-[4]吡啶基)-胺
硝乙醛肟