(2E,4E)-octa-2,4-diene | 60919-80-4
中文名称
——
中文别名
——
英文名称
(2E,4E)-octa-2,4-diene
英文别名
trans-2,4-octadiene;2,4-octadiene;2,4-Octadien;octa-2t,4t-diene;(E,E)-2,4-Octadien
CAS
60919-80-4
化学式
C8H14
mdl
——
分子量
110.199
InChiKey
NZLCAHVLJPDRBL-VSAQMIDASA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:130.8±7.0 °C(Predicted)
-
密度:0.745±0.06 g/cm3(Predicted)
-
保留指数:811
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
反应信息
-
作为反应物:描述:马来酸酐 、 (2E,4E)-octa-2,4-diene 以 苯 为溶剂, 反应 4.0h, 以45 mg的产率得到(+/-)-(3aS,4S,7R,7aR)-4-methyl-7-n-propyl-3a,4,7,7a-tetrahydrobenzo
furan-1,3-dione 参考文献:名称:Cocker, Wesley; Geraghty, Niall W. A.; Shannon, Patrick V. R., Journal of the Chemical Society. Perkin transactions I, 1984, # 10, p. 2241 - 2244摘要:DOI: -
作为产物:描述:参考文献:名称:Cocker, Wesley; Geraghty, Niall W. A.; Shannon, Patrick V. R., Journal of the Chemical Society. Perkin transactions I, 1984, # 10, p. 2241 - 2244摘要:DOI:
文献信息
-
Novel<i>ortho</i>-Alkoxy-Substituted Phosphorus Ylides and Their Stereoselectivity in Witting Reactions作者:Suruliappa Jeganathan、Masamitsu Tsukarmoto、Manfred SchlosserDOI:10.1055/s-1990-26801日期:——The stereochemistry of the reactions between tris(2-methoxy-methoxyphenyl)phosphonioethanide (1f), -butanide (2f), and -phenyl-methanide (3f) and a variety of aldehydes was investigated. Ylides having a β-unbranched aliphatic sidechain, such as 2f, and saturated straight-chain aldehydes give olefins with unprecedented cis-selectivity (cis/trans â 200:1).
-
Enantioselective Rare-Earth Catalyzed Quinone Diels−Alder Reactions作者:David A. Evans、Jimmy WuDOI:10.1021/ja0367602日期:2003.8.1A highly enantioselective, quinone Diels-Alder reaction catalyzed by chiral samarium and gadolinium pyridyl-bis(oxazoline) (pybox) complexes has been developed. The reaction scope has been extended to include three quinones and five dienes, all of which exclusively provide the expected endo product in excellent yields and enantioselectivities.
-
Method for the preparation of sulfones and compounds containing carbon申请人:The Research Foundation of State University of New York公开号:US04604480A1公开(公告)日:1986-08-05A method for the preparation of a first sulfone compound of the formula: ##STR1## wherein R.sub.a is ##STR2## where R.sub.b is Br and R.sub.c is H except that R.sub.b and R.sub.c together may be an electron pair when R.sub.6 is a radical of the formula: ##STR3## wherein X.sub.1 is independently chlorine, bromine or iodine and R.sub.1 and R.sub.2 are independently at each occurrence hydrogen or, substituted or unsubstituted, phenyl or alkyl where the substituents are halogen or alkoxy or additional --SO.sub.2 Br groups; provided that, each carbon atom of R.sub.1 or R.sub.2 which contains --SO.sub.2 Br also contains an X.sub.1 group and wherein R.sub.3 through R.sub.9 are independently --OZ,--C.sub.6 M.sub.5,--Z,--SiZ.sub.3 or --X.sub.2, where Z is hydrogen or substituted or unsubstituted phenyl, alkyl, alkenyl or alkynyl; X.sub.2 is chlorine, bromine, iodine or fluorine; M is independently at each occurrence Z or X.sub.2 ; R.sub.3 and R.sub.4 may together be an electron pair; two or more of R.sub.3, R.sub.4, R.sub.5 and R.sub.6 may be combined together and with one or more of C.sub.2, C.sub.3 or C.sub.4 to form a ring structure and R.sub.1 and R.sub.2 may be joined together with C.sub.1 to form a ring structure; said method comprising reacting a 1-haloalkyl 1-sulfonyl halide with a second compound of the formula: ##STR4## at a temperature below 25.degree. C. for less than 12 hours where R.sub.3, R.sub.4 and R.sub.5 are as previously described, R.sub.10 is R.sub.6 as previously described or R.sub.11, a radical of the formula: ##STR5## This invention was made with Government support under CHE 811530801 awarded by the National Science Foundation. The Government has certain rights in this invention.一种制备第一砜化合物的方法,其化学式为:其中R.sub.a为##STR2##其中R.sub.b为Br,R.sub.c为H,但当R.sub.6为以下式的基团时,R.sub.b和R.sub.c可能一起是一个电子对:##STR3##其中X.sub.1独立地是氯、溴或碘,R.sub.1和R.sub.2在每次出现时独立地是氢或取代或未取代的苯或烷基,其中取代基是卤素或烷氧基或额外的--SO.sub.2 Br基团;条件是,R.sub.1或R.sub.2的每个含--SO.sub.2 Br的碳原子也含有一个X.sub.1基团,R.sub.3到R.sub.9独立地是--OZ,--C.sub.6 M.sub.5,--Z,--SiZ.sub.3或--X.sub.2,其中Z是氢或取代或未取代的苯、烷基、烯基或炔基;X.sub.2是氯、溴、碘或氟;M在每次出现时独立地是Z或X.sub.2;R.sub.3和R.sub.4可能一起是一个电子对;R.sub.3、R.sub.4、R.sub.5和R.sub.6中的两个或更多个可能结合在一起,并与C.sub.2、C.sub.3或C.sub.4中的一个或多个结合形成环结构,R.sub.1和R.sub.2可能与C.sub.1结合形成环结构;所述方法包括将1-卤代烷基-1-磺酰卤化物与以下式的第二化合物反应:在低于25°C的温度下,反应时间少于12小时,其中R.sub.3、R.sub.4和R.sub.5如前所述,R.sub.10为如前所述的R.sub.6或R.sub.11,即以下式的基团:##STR5##此发明是在国家科学基金会授予的CHE 811530801号下获得政府支持的。政府对该发明拥有特定的权利。
-
Lehmkuhl, Herbert; Fustero, Santos, Liebigs Annalen der Chemie, 1980, # 9, p. 1353 - 1360作者:Lehmkuhl, Herbert、Fustero, SantosDOI:——日期:——
-
Use of α-chlorosulfides in indium promoted CC couplings: easy entry into the stereoselective formation of epoxy alkynes作者:Gregory Engstrom、Meisha Morelli、Carolyn Palomo、Thomas MitzelDOI:10.1016/s0040-4039(99)01152-1日期:1999.8The use of alpha-chlorosulfide compounds to control stereoselectivity in indium promoted C-C couplings occurs smoothly at room temperature under aqueous and mixed aqueous/organic conditions. Use of the halide to control syn/anti ratios simplifies the indium promoted coupling with respect to earlier studies and is used to gain entry into stereocontrolled epoxy alkynes in good yields. (C) 1999 Elsevier Science Ltd. All rights reserved.
表征谱图
-
氢谱1HNMR
-
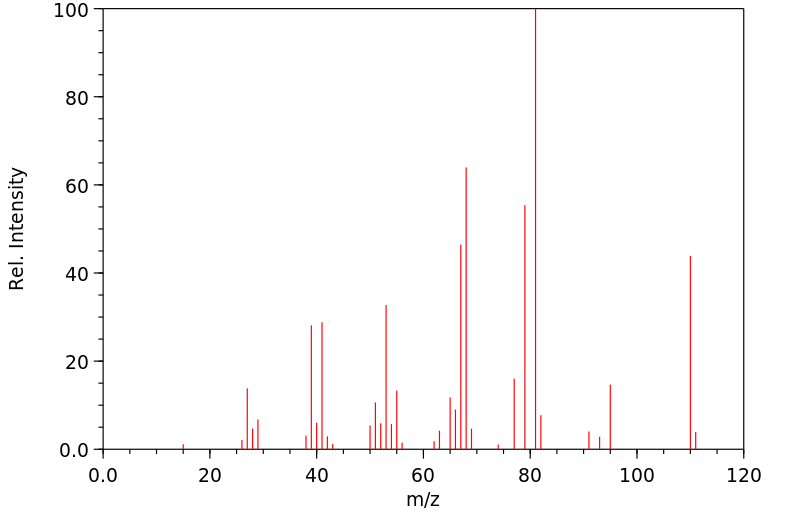
质谱MS
-
碳谱13CNMR
-
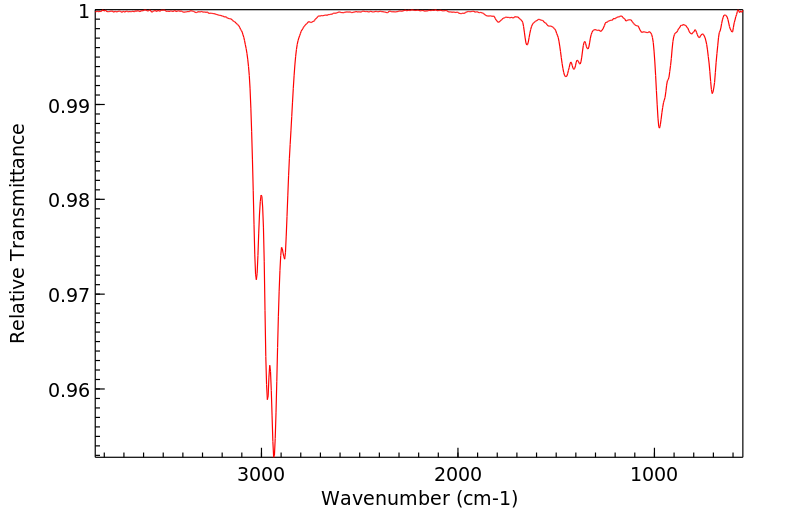
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-